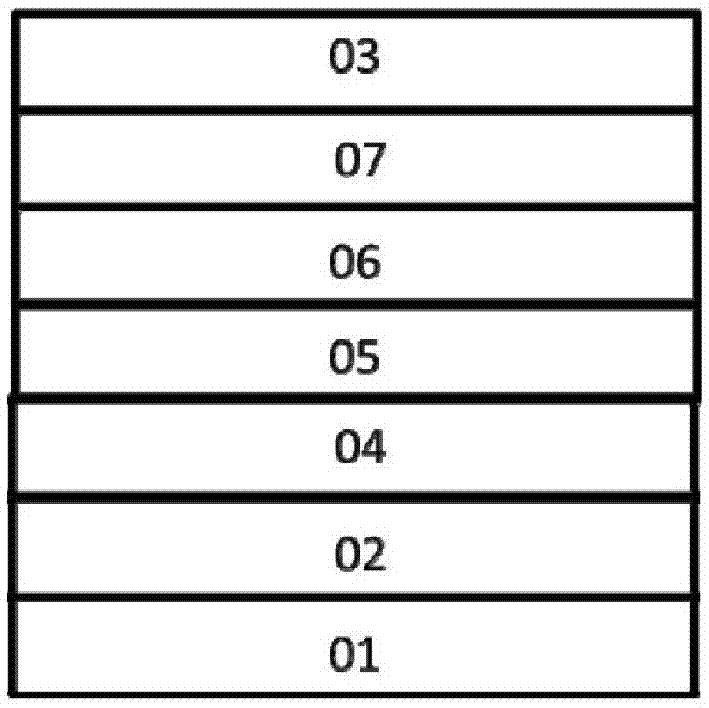
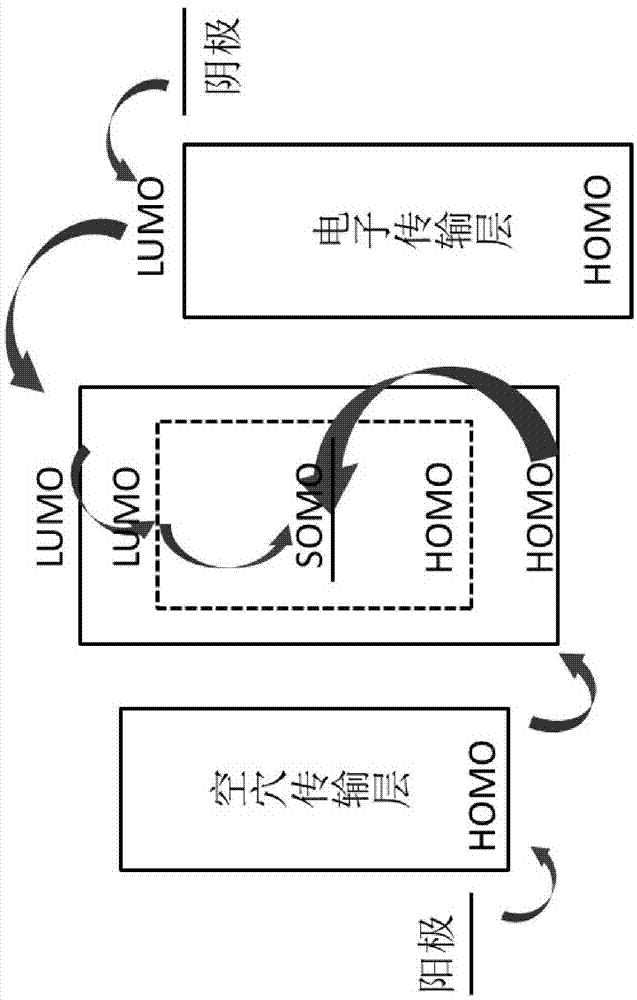
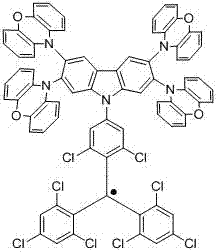
Organic electroluminescent device
An electroluminescent and organic technology, applied in the field of organic electroluminescent devices, can solve the problems of inability to meet production requirements, low efficiency, and unstable performance of phosphorescent devices.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0131]
[0132] Synthesize the compound shown in formula (1-1): TTM (1.81 mmol), 2,3,6,7-tetracarbazolylcarbazole (9.0 mmol), anhydrous Cs 2 CO 3 (2.9 mmol), and a mixture of DMF (20 mL) was stirred at 160°C for 2.5 hours. The reacted mixture was cooled to room temperature, the product was filtered with 1M concentrated hydrochloric acid, and purified by suction filtration, and then the crude product was purified by column chromatography (petroleum ether: dichloromethane = 5:1 v / v) to obtain formula (1-1) , yield 40%.
[0133] The molecular weight obtained by mass spectrometry: 1402.96.
[0134] The relative molecular mass percentage of each element obtained by elemental analysis: C: 71.06; H: 3.74; Cl: 20.22; N: 4.99.
Embodiment 2
[0136] Synthesis of compounds of the structure shown in formula (1-2): The reactant 2,3,6,7-tetracarbazolylcarbazole is replaced by (3,6-di-tert-butyl)-2,3,6,7-tetra Carbazole-carbazole, through the same synthesis method as in Example 1, the compound with the structure shown in formula (1-2) was obtained with a yield of 39%.
[0137] The molecular weight obtained by mass spectrometry: 1835.77.
[0138] The relative molecular mass percentage of each element obtained by elemental analysis: C: 74.59; H: 6.15; Cl: 15.45; N: 3.81.
Embodiment 3
[0140] Synthesis of compounds of the structure shown in formula (1-3): The reactant 2,3,6,7-tetracarbazolylcarbazole is replaced by (3,6-dimethyl ether)-2,3,6,7-tetra Carbazole-carbazole, through the same synthesis method as in Example 1, the compound with the structure shown in formula (1-3) was obtained with a yield of 35%.
[0141] The molecular weight obtained by mass spectrometry: 1626.22.
[0142] The relative molecular mass percentage of each element obtained by elemental analysis: C: 66.43; H: 3.96; Cl: 17.43; N: 4.30; O: 7.87.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com