Red light black active dye of azo anthraquinone mixing light emitting system containing iso-bifunctional groups with high color fixation rate, and preparation method and application of red light black active dye
A dual reactive group, reactive dye technology, applied in reactive dyes, azo dyes, dyeing methods, etc., can solve the problems of molar extinction coefficient, low color fixation rate, poor dye depth, etc., and achieve good color fixation rate and bright color. , Improve the effect of dye utilization
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
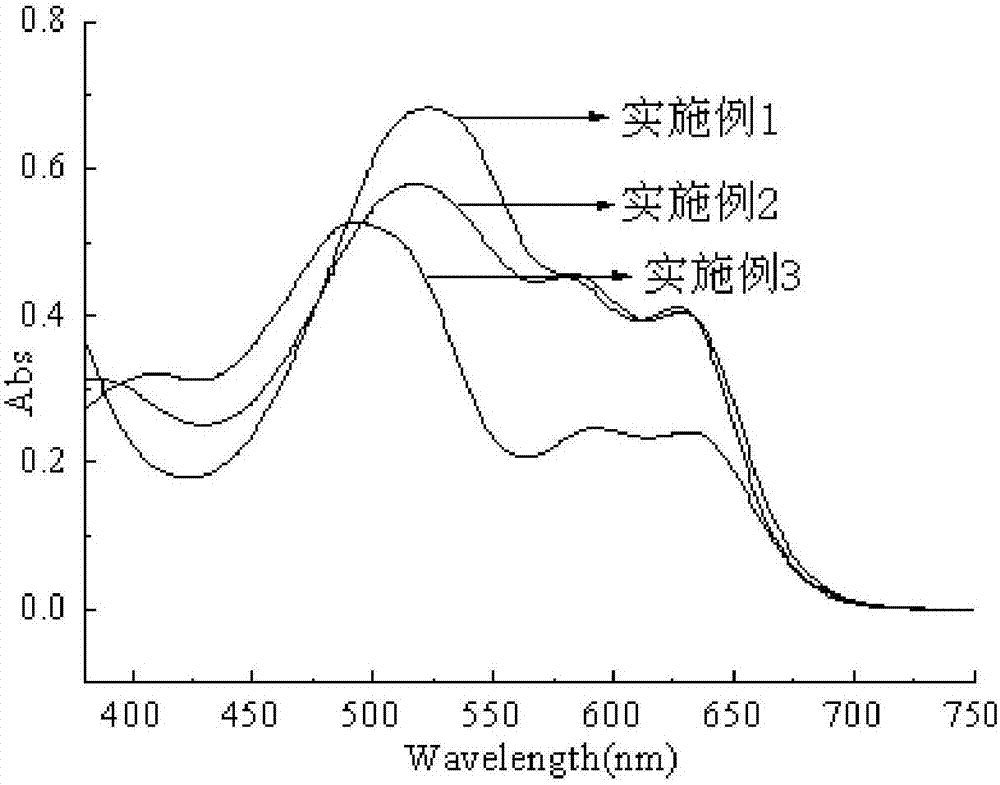
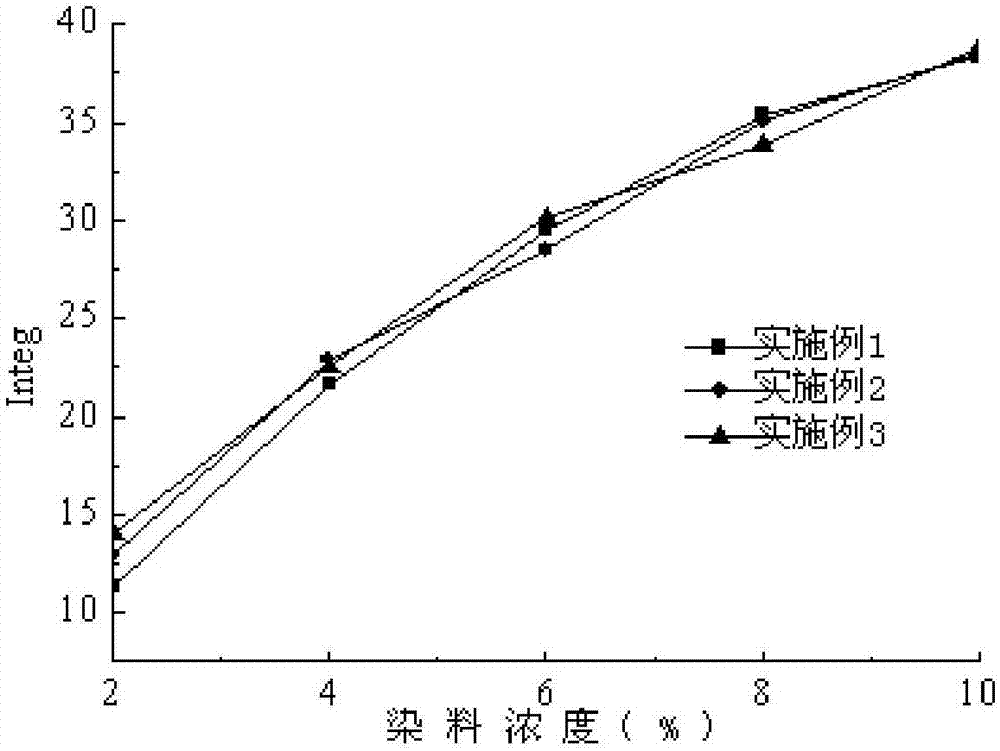
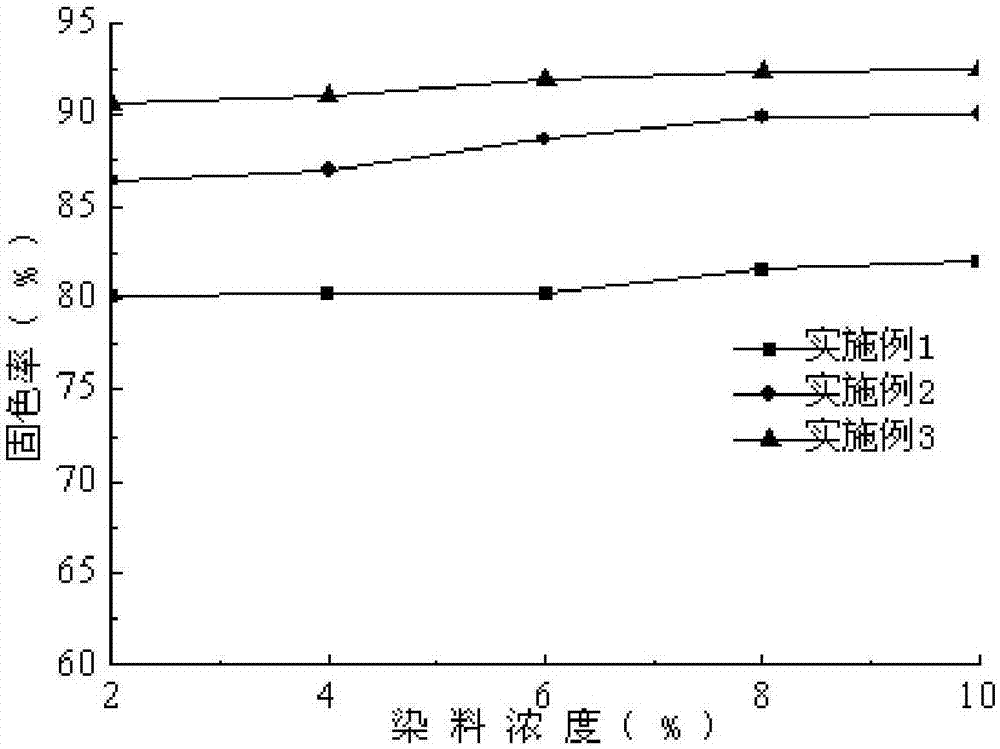
Image
Examples
Embodiment 1
[0034] The structural formula and preparation method of the red light black reactive dye of the heterobiactive group azoanthraquinone mixed chromogenic system prepared in this example are as follows:
[0035]
[0036] (1) Preparation of a condensate: 18.6g (0.1mol) of cyanuric chloride was beaten in 80g of ice-water mixture for 1 hour at 0-2°C. Accurately weigh 54.7g (0.099mol) of 1-amino-2-sulfonic acid-4-(3-amino-2,4,6-trimethyl-5-sulfonic acid phenylamino)anthraquinone monosodium salt to prepare 15wt % aqueous solution, dissolved into a blue clear solution, added dropwise to the uniformly beaten cyanuric chloride at 0°C within 1 hour, after the dropwise addition, keep the temperature at 5°C, and react for 5 hours with a pH value of 3 to 3.5. The end point of the shrinkage reaction was detected by layer chromatography.
[0037] (2) Preparation of dicondensate: Add 67.1g (0.197mol) of 1-amino-8-naphthol-3,6-disulfonic acid dry powder into a dicondensate, adjust the pH val...
Embodiment 2
[0041] The structural formula and preparation method of the red light black reactive dye of the heterobiactive group azoanthraquinone mixed chromogenic system prepared in this example are as follows:
[0042]
[0043](1) Preparation of a condensate: 18.6g (0.1mol) of cyanuric chloride was beaten in 80g of ice-water mixture for 1 hour at 0-2°C. Accurately weigh 54.7g (0.099mol) of 1-amino-2-sulfonic acid-4-(3-amino-2,4,6-trimethyl-5-sulfonic acid phenylamino)anthraquinone monosodium salt to prepare 15wt % aqueous solution, dissolved into a blue clear solution, added dropwise to the uniformly beaten cyanuric chloride at 0°C within 1 hour, kept at 0°C after the dropwise addition, and reacted for 5 hours with a pH value of 3 to 3.5, thin The end point of the shrinkage reaction was detected by layer chromatography. Simultaneously, the shrinkage reaction of 18.6g (0.1mol) cyanuric chloride and 27.8g (0.099mol) p-(β-ethylsulfone sulfone group) aniline is carried out simultaneousl...
Embodiment 3
[0048] The structural formula and preparation method of the red light black reactive dye of the heterobiactive group azoanthraquinone mixed chromogenic system prepared in this example are as follows:
[0049]
[0050] (1) Preparation of a condensate: 18.6g (0.1mol) of cyanuric chloride was beaten in 80g of ice-water mixture for 1 hour at 0-2°C. Accurately weigh 54.7g (0.099mol) of 1-amino-2-sulfonic acid-4-(3-amino-2,4,6-trimethyl-5-sulfonic acid phenylamino)anthraquinone monosodium salt to prepare 15wt % aqueous solution, dissolved into a blue clear solution, added dropwise to the uniformly beaten cyanuric chloride at 0°C within 1 hour, after the dropwise addition, keep the temperature at 5°C, and react for 5 hours with a pH value of 3 to 3.5. The end point of the shrinkage reaction was detected by layer chromatography. Simultaneously, the shrinkage reaction of 18.6g cyanuric chloride and 27.8g (0.099mol) p-(β-sulfone ethylsulfone group) aniline is carried out simultaneou...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com