Rabies virus inhibition polypeptide as well as preparation method and application thereof
A technology of rabies virus and inhibitory polypeptide, which is applied in the fields of biochemistry and molecular biology, can solve the problems of lack of safe and efficient intracellular transport system, low toxicity, and no solution, so as to inhibit virus replication, strong pertinence, and inhibition Obvious effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] Example 1 optimizes the design of the rabies virus inhibitory polypeptide sequence by QSAR
[0029] (1) From published literature (Real E, Rain JC, Battaglia V, Jallet C, Perrin P, Tordo N, Chrisment P, D'Alayer J, Legrain P, Jacob Y. Antiviral drug discovery strategy using combinatorial libraries of structurally constrained peptides .JVirol.2004Jul; 78(14):7410-7.) has collected the peptide data that has been confirmed to inhibit the replication of rabies virus for modeling, a total of 29 polypeptides, and the sequences are shown in Table 1:
[0030] Table 1 Sequence information used in QSAR modeling
[0031]
[0032]
[0033] (2) The descriptor variables ( http: / / bidd2.nus.edu.sg / cgi-bin / profeat2016 / main.cgi ), four descriptor combinations of G3, G4, G5, and G9 were selected for calculation; the genetic algorithm (Genetic algorithm, GA) was used to screen the physical and chemical parameters directly related to the biological activity of the polypeptide, and...
Embodiment 2
[0040] Example 2 Activity Verification of Starting Peptides and Optimizing Peptides
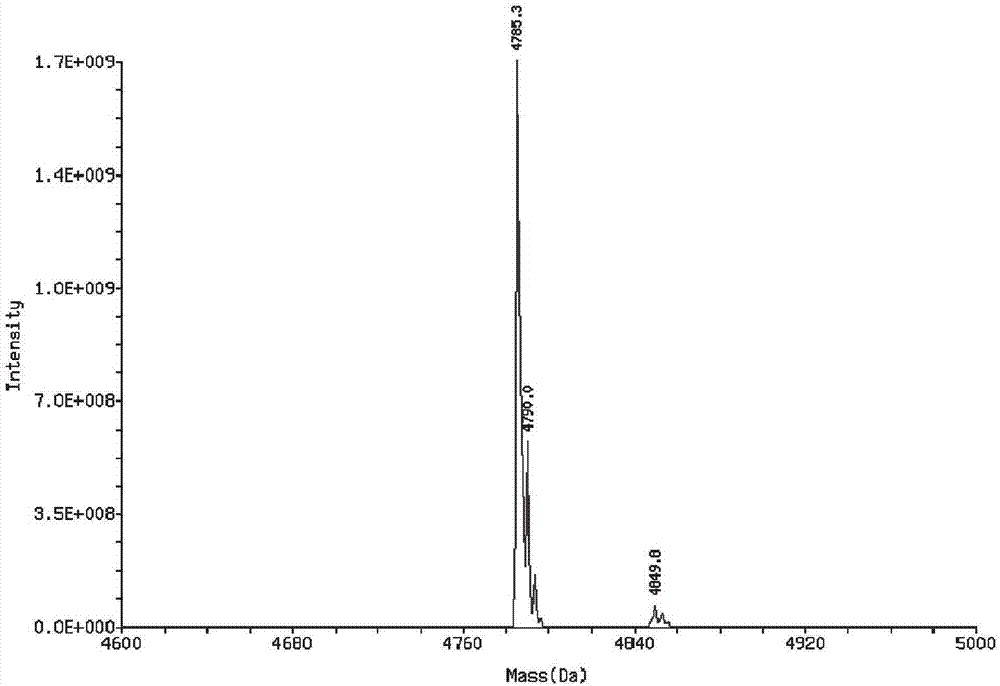
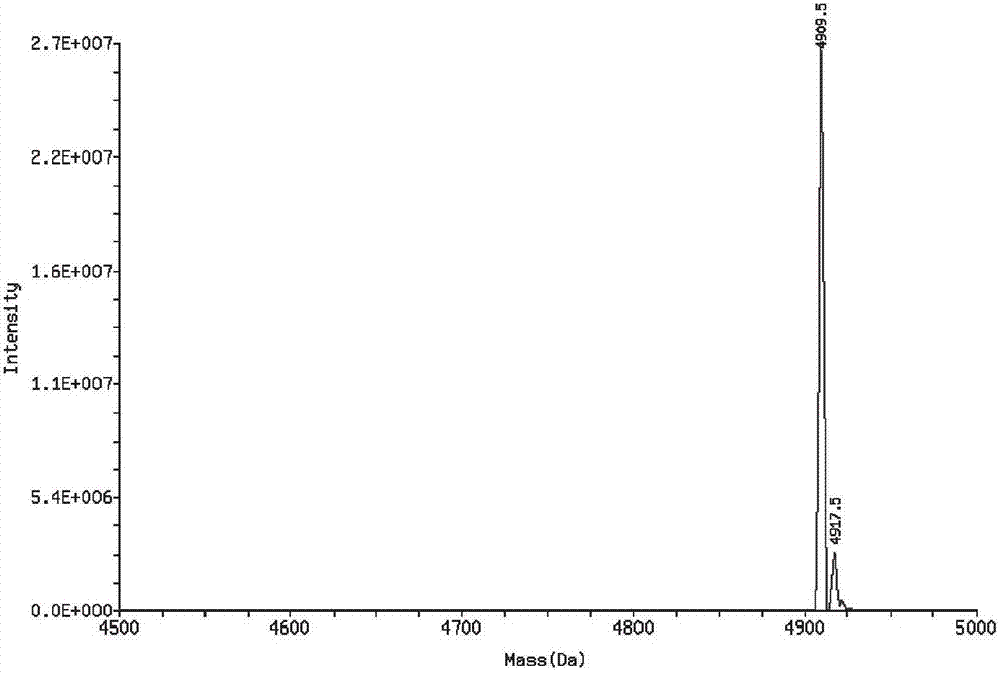
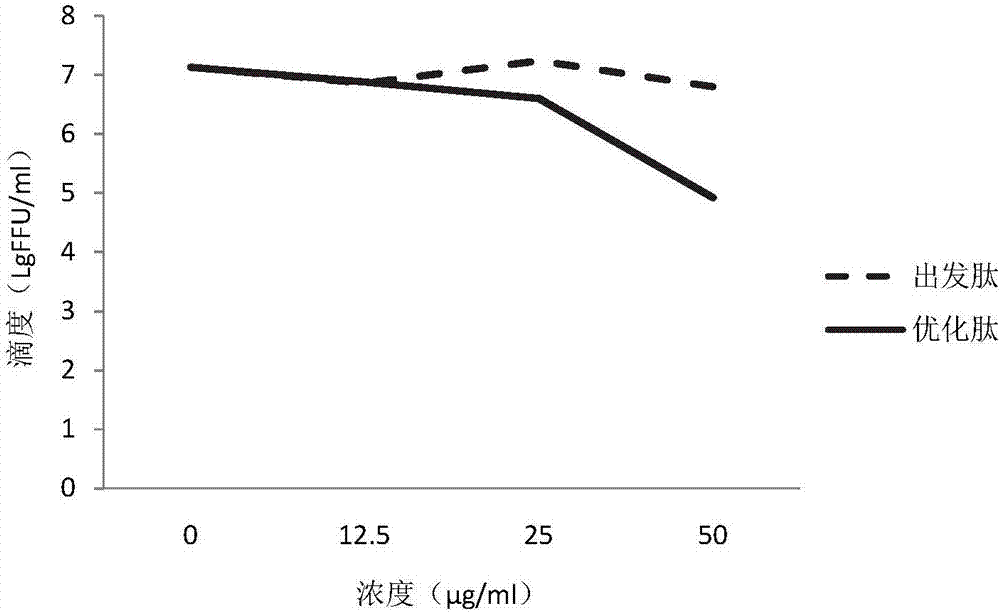
[0041]The starting peptide and optimized peptide in Example 1 were synthesized by solid-phase synthesis. In order to solve the problem of poor cell permeability of polypeptide drugs, a membrane-penetrating peptide sequence RRRRRRRRR was connected to the N-terminals of the starting peptide and the optimized peptide respectively. The mass spectra of the synthesized starting peptide with membrane-penetrating peptide and the optimized fetus with membrane-penetrating peptide are shown in figure 1 , 2 , the sequences are:
[0042] SEQ ID No. 50: RRRRRRRR PPDVHTPPHALWRLHLSLRVCLVRMWIH
[0043] SEQ ID No. 51: RRRRRRRRRPPDVHTPPHALWRLHLILRVELVRMWWH.
[0044] Prepare 8 groups of BSR cells in parallel, inoculate BSR cells with 0.01 MOI of CVS, and add different concentrations of starting peptide and optimized peptide drug 1 h later (final concentrations are 0 μg / ml, 12.5 μg / ml, 25 μg / ml, 50 μg / ml, res...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com