Levofloxacin aldehyde acetal 4-aryl thiosemicarbazide derivatives and its preparation method and application
A technology of levofloxacin and levofloxacin hydrazide, applied in the fields of new drug discovery and innovative drug synthesis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
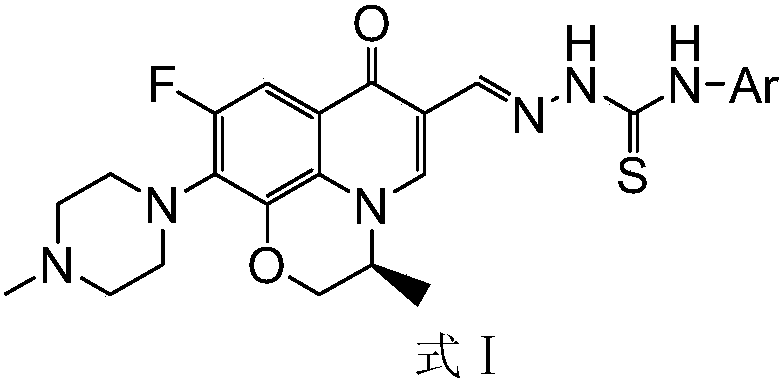
[0034] (S)-6-fluoro-7-(4-methyl-piperazin-1-yl)-1,8-(2,1-oxopropyl)-quinolin-4(1H)-one-3- Aldehyde 4-phenylthiosemicarbazide (I-1), its chemical structural formula is:
[0035]
[0036] That is, Ar in formula I is a benzene ring.
[0037] The preparation method of this compound is: levofloxacin aldehyde crude product (1.0g) shown in formula IV is dissolved in absolute ethanol (30 milliliters), adds 4-phenylthiosemicarbazide (0.6g, 3.6mmol) shown in formula VIII, Reflux for 12 hours, filter while hot, wash 2 times with ethanol, wash the solid twice with distilled water successively, dry, and recrystallize with DMF-ethanol (V:V=5:3) mixed solvent to obtain light yellow crystals of formula (I -1), obtain product 0.63g, m.p.234~236 ℃. 1 H NMR (400MHz, DMSO-d 6 ): 11.76(s, 1H, CH=N), 9.95(s, 1H, NH), 8.87(s, 1H, 2-H), 8.45(s, 1H, NH), 7.62~7.25(m, 6H, Ph-H and5-H),4.61~4.34(m,3H,OCH 2 CH), 3.24(t, 4H, piperazine-H), 2.45(t, 4H, piperazine-H), 2.23(s, 3H, N-CH 3 ), 1.45 (d,...
Embodiment 2
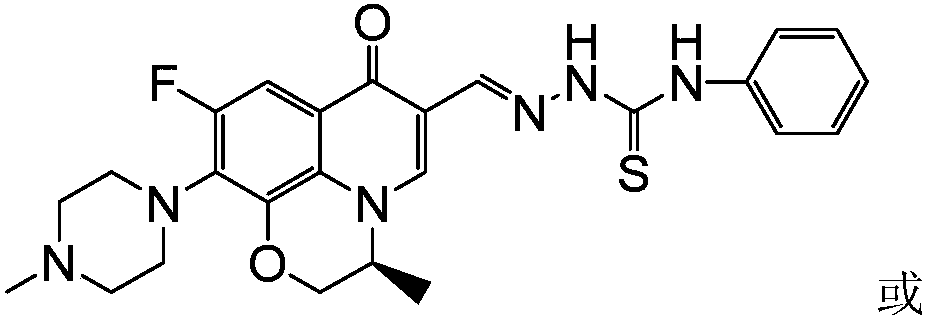
[0039] (S)-6-fluoro-7-(4-methyl-piperazin-1-yl)-1,8-(2,1-oxopropyl)-quinolin-4(1H)-one-3- Aldehyde 4-(4-methylphenyl) thiosemicarbazide (I-2), its chemical structural formula is:
[0040]
[0041] That is, Ar in formula I is 4-methylphenyl.
[0042]The preparation method of this compound is: levofloxacin aldehyde crude product (1.0g) shown in formula IV is dissolved in absolute ethanol (30 milliliters), adds 4-(4-methylphenyl) thiosemicarbazide (0.6g) shown in formula VIII ,3.3mmol), reflux reaction for 10 hours, filtered while hot, the solid was washed twice with ethanol and distilled water twice, dried, and recrystallized with a mixed solvent of DMF-ethanol (V:V=5:3) to obtain light yellow Crystalline formula (I-2), 0.58g of the product was obtained, m.p.227-229°C. 1 H NMR (400MHz, DMSO-d 6 ): 11.77(s, 1H, CH=N), 9.86(s, 1H, NH), 8.91(s, 1H, 2-H), 8.44(s, 1H, NH), 8.25~7.46(m, 5H, Ph-H and5-H),4.63~4.35(m,3H,OCH 2 CH), 3.24(t, 4H, piperazine-H), 2.42(t, 4H, piperazin...
Embodiment 3
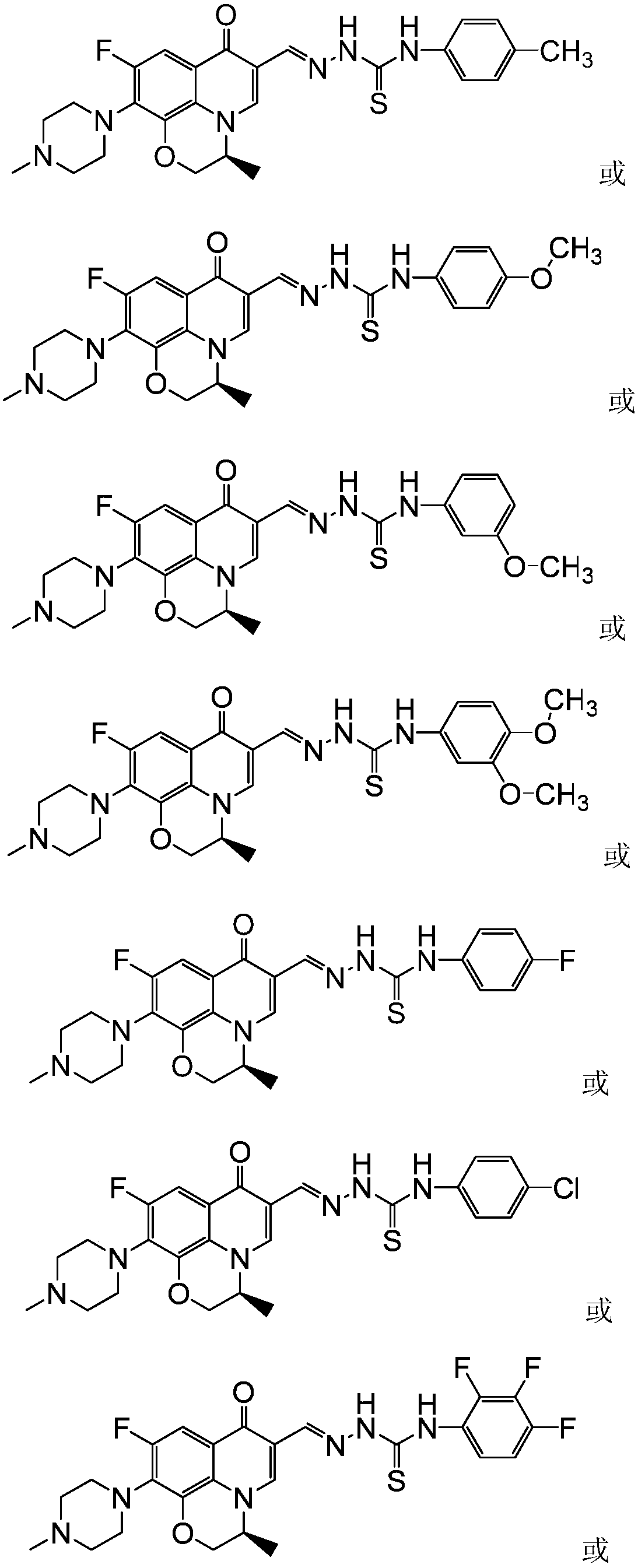
[0044] (S)-6-fluoro-7-(4-methyl-piperazin-1-yl)-1,8-(2,1-oxopropyl)-quinolin-4(1H)-one-3- Aldehyde 4-(4-methoxyphenyl) thiosemicarbazide (I-2), its chemical structural formula is:
[0045]
[0046] That is, Ar in formula I is 4-methoxyphenyl.
[0047] The preparation method of this compound is: levofloxacin aldehyde crude product (1.0g) shown in formula IV is dissolved in dehydrated alcohol (30 milliliters), adds 4-(4-methoxyphenyl) thiosemicarbazide shown in formula VIII ( 0.7g, 3.6mmol), reflux reaction for 10 hours, filtered while hot, the solid was washed twice with ethanol and distilled water twice, dried, and recrystallized with DMF-ethanol (V:V=5:3) mixed solvent to obtain Pale yellow crystal formula (I-3), 0.65 g of the product, m.p.235-237°C. 1 H NMR (400MHz, DMSO-d 6 ): 11.75(s, 1H, CH=N), 9.87(s, 1H, NH), 8.87(s, 1H, 2-H), 8.43(s, 1H, NH), 7.48~6.95(m, 5H, Ph-H and5-H),4.58~4.33(m,3H,OCH 2 CH), 3.78(s,3H,OCH 3 ), 3.24(t, 4H, piperazine-H), 2.45(t, 4H, piper...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com