Fluoride-free super-hydrophobic coating and preparation method thereof
A technology of super-hydrophobic coatings and nanomaterials, which is applied in the field of fluorine-free super-hydrophobic coatings and its preparation, can solve the problems of high cost, and achieve the effects of low cost, easy availability of raw materials, and good mechanical properties of the coating
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
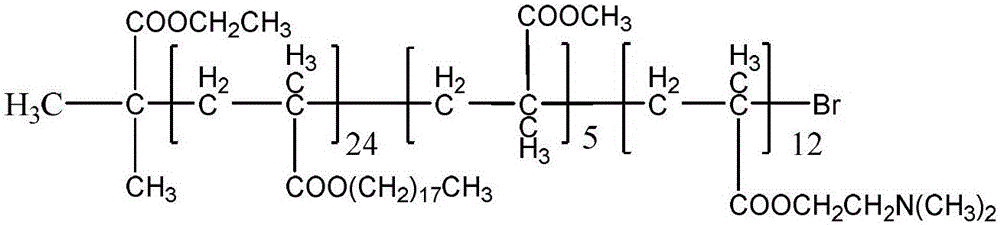
[0029] Example 1: The functional polyacrylate block copolymer has a molecular weight of 10500 and a structure of:
[0030]
[0031] A. Preparation of functional long side chain polyacrylate block copolymer:
[0032] Add octadecyl methacrylate (OMA) 16.90 g (0.05 mol), methyl methacrylate (MMA) 1.05 g (0.01 mol), initiator α-bromoisobutyric acid ethyl in a 500 ml four-necked flask Ester (EBiB) 0.4120g (0.0021mol), catalyst CuBr 2 0.0061g (2.5×10 -5 mol), ligand PMDETA0.0433g (2.5×10 -4 mol), stannous octoate (Sn(EH) 2 )0.1519g (3.75×10 -4mol), solvent 1-methyl-2-pyrrolidone 12.0g, after mixing uniformly, nitrogen gas was passed for 1h, and reacted in 75°C oil bath for 5.5h, and the conversion rate reached 80%, and a white viscous product was obtained. Then, 4.20 g (0.0268 mol) of N,N dimethylaminoethyl methacrylate (DMAEMA) was added into the reaction vessel, and the reaction was continued for 2 hours to obtain a functional polyacrylate block copolymer.
[0033] B. Pre...
Embodiment 2
[0037] Example 2: The functional polyacrylate block copolymer has a molecular weight of 12500 and a structure of:
[0038]
[0039] A. Preparation of functional polyacrylate block copolymer:
[0040] In a 500ml four-necked flask, add octadecyl acrylate (OA) 16.90g (0.05mol), ethyl methacrylate (EMA) 1.20g (0.011mol), initiator α-bromo ethyl isobutyrate ( EBiB) 0.3296g (0.0017mol), catalyst CuBr 2 0.0092g (4.11×10 -5 mol), ligand PMDETA0.0734g (4.25×10 -4 mol), stannous octoate (Sn(EH) 2 )0.3038g (7.5×10 -4 mol), solvent toluene 10.0g, after mixing uniformly, nitrogen gas was passed for 1h, and reacted in 72°C oil bath for 5.5h, and the conversion rate reached 80%, and a white viscous product was obtained. Then, 3.40 g (0.022 mol) of N,N dimethylaminoethyl methacrylate (DMAEMA) was added into the reaction vessel, and the reaction was continued for 5 hours to obtain a functional polyacrylate block copolymer.
[0041] B. Preparation of Hydrophobic Particles
[0042] 2.5...
Embodiment 3
[0045] Embodiment 3: The molecular weight of the functional polyacrylate block copolymer is 14500, and the structure is:
[0046]
[0047] A. Preparation of functional polyacrylate block copolymer:
[0048] Add hexadecyl acrylate (HDMA) 15.24g (0.05mol), ethyl methacrylate (EMA) 1.93g (0.017mol) in the 500ml four-necked flask, initiator α-bromo ethyl isobutyrate ( EBiB) 0.3296g (0.0017mol), catalyst CuBr 2 0.0061g (2.5×10 -5 mol), ligand PMDETA0.0433g (2.5×10 -4 mol), stannous octoate (Sn(EH) 2 )0.3038g (7.5×10 -4 mol), solvent anisole 15.0g, after mixing uniformly, nitrogen gas was passed for 1h, and then reacted in 75°C oil bath for 6.0h, after the conversion rate reached 80%, a white viscous product was obtained. Then, 7.72 g (0.054 mol) of N,N dimethylaminoethyl acrylate (DMAEA) was added into the reaction vessel, and the reaction was continued for 4 hours to obtain a functional polyacrylate block copolymer.
[0049] B. Preparation of Hydrophobic Particles
[005...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com