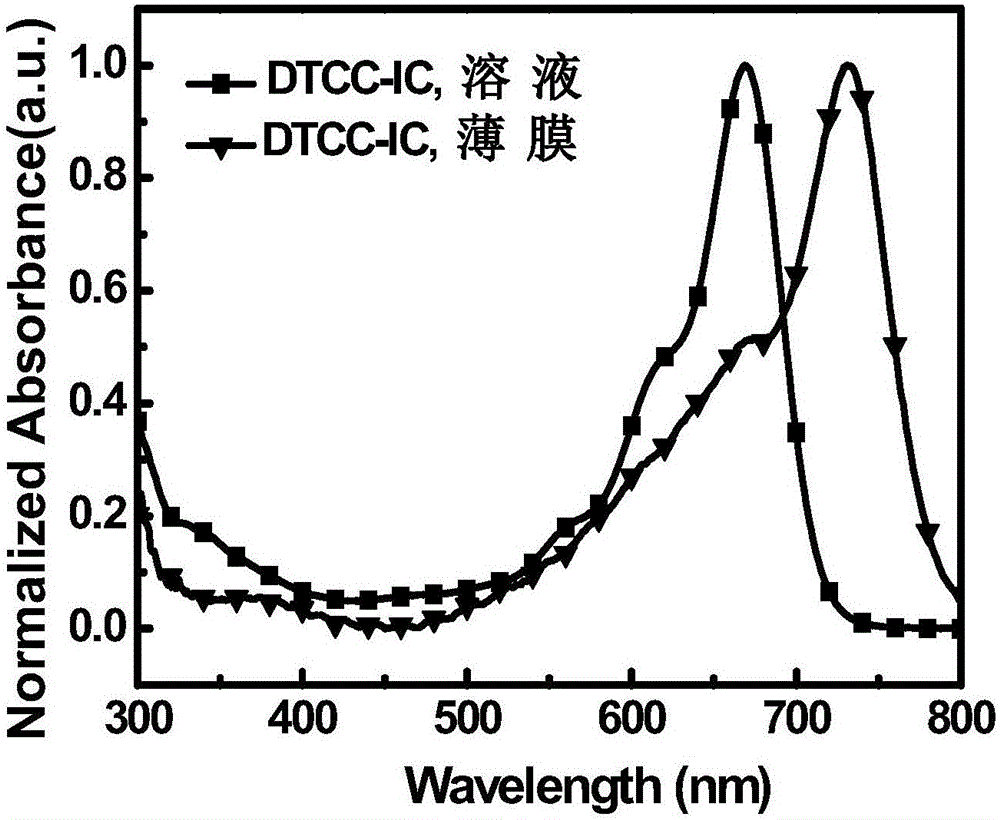
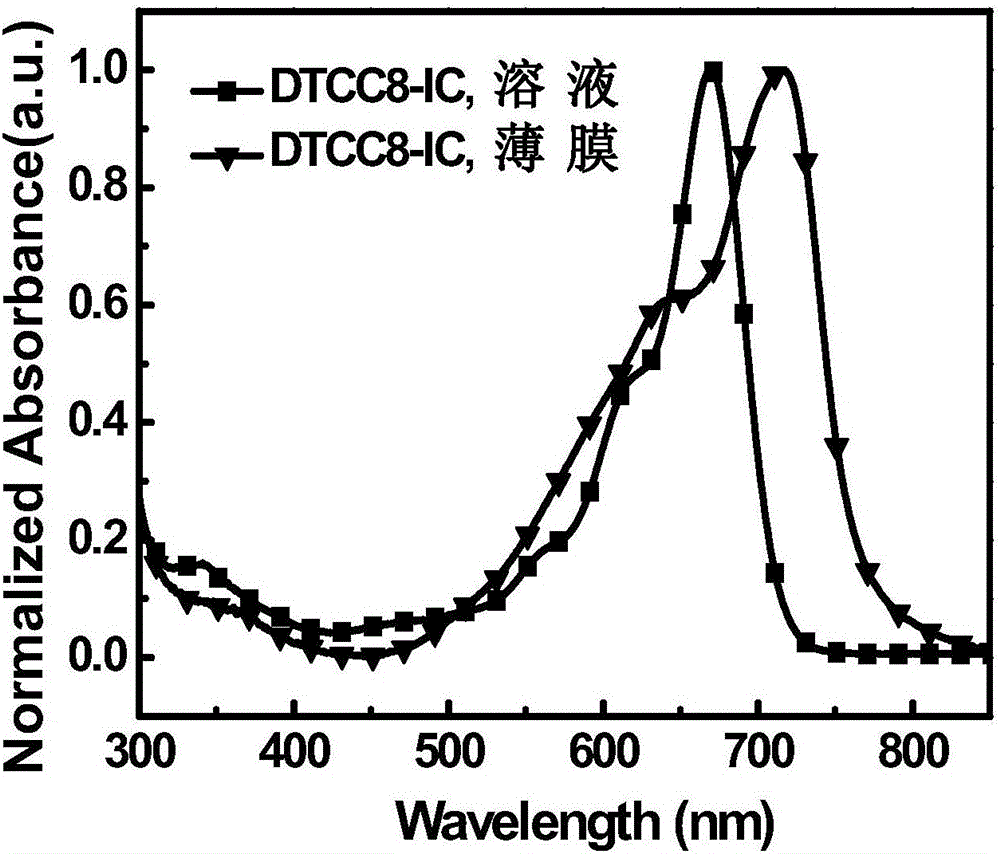
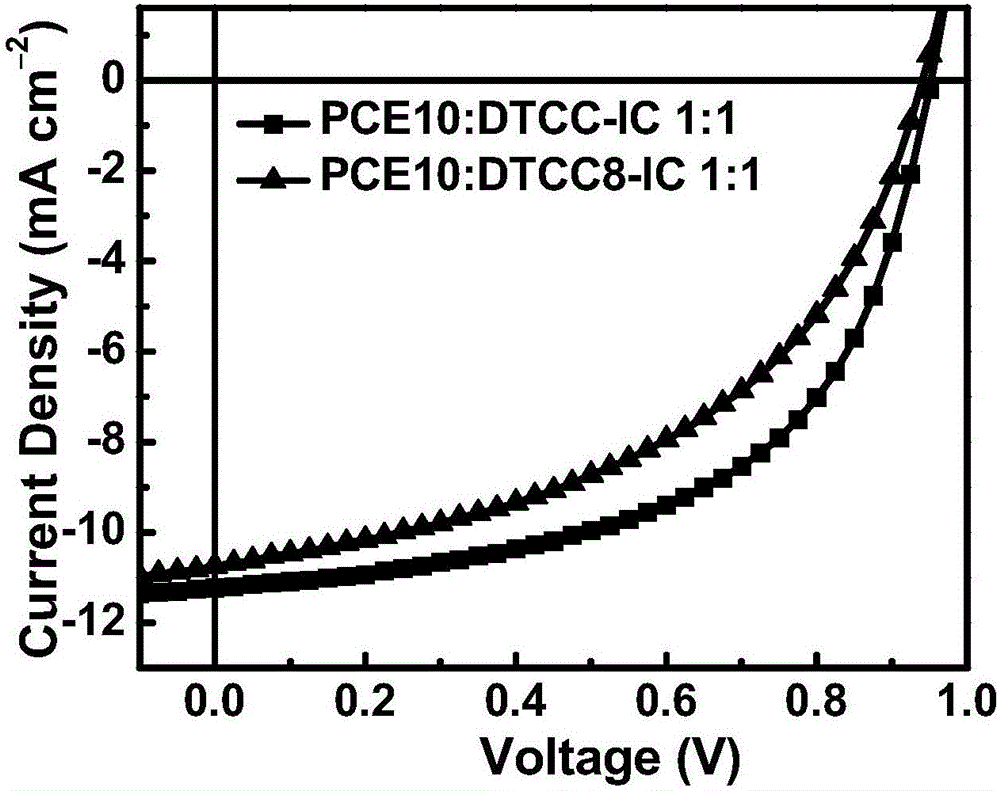
A-D-A type organic small molecular receptor containing thienocarbazole seven-membered condensed ring and preparation method thereof
A technology of small molecule receptors and carbazoles, which is applied in the field of organic small molecule receptor materials and their preparation, can solve the problems that n-type organic small molecule receptor materials have not been reported, and achieve the availability and popularization of raw materials High, synthetically simple effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0053] A kind of organic small molecule acceptor whose chemical structural formula is DTCC-IC, its synthetic route is as follows:
[0054]
[0055] (1) The chemical structural formula is the synthesis of the intermediate of a: with (2.7g, 5.1mmol) carbazole borate ester, (3.7g, 15.3mmol) thiophene ester halogenated compound, (13.8g, 100.0mmol) anhydrous K 2 CO 3 , an appropriate amount of water, 2 drops of tetrabutylammonium bromide, 0.5g of palladium catalyst, and 100mL of toluene were added to the reaction flask. Protected under nitrogen, protected from light, reacted at 100°C for 24 hours. Cool to room temperature, extract with dichloromethane and water, anhydrous MgSO 4 Dried, spin-dried and passed through the column to obtain a yellow solid, namely intermediate a, with a yield of 75%. 1 H NMR (400MHz, CDCl 3 ),δ(ppm):8.08–8.10(d,2H),7.54–7.56(m,4H),7.36–7.38(dd,2H),7.27–7.28(d,2H),4.30(t,2H), 4.17–4.22(m,4H),1.85–1.91(m,2H),1.23–1.42(m,10H),1.12–1.16(t,3H),0.82–0....
Embodiment 2
[0060] The synthetic route of the small organic molecule receptor whose chemical structural formula is DTCC8-IC is as follows:
[0061]
[0062] (1) chemical structural formula is the synthesis of the intermediate of a with embodiment 1
[0063] (2) Synthesis of an intermediate whose chemical structural formula is e: with (0.6g, 25mmol) magnesium chips, an appropriate amount of elemental iodine as a catalyst, (5.0g, 18.6mmol) p-bromooctylbenzene, 120mL THF, join the reaction flask middle. Under nitrogen protection, it was initiated at 100°C. After reacting at this temperature for 12 hours, (1.5 g, 2.6 mmol) THF solution of intermediate a was added into the reaction system at room temperature, and then the temperature was raised to 110° C. and stirred for 12 hours. Cool to room temperature, extract with dichloromethane and water, wash over anhydrous MgSO 4 After being dried and spin-dried through the column, 100 mL of acetic acid was added, and stirred at 100° C. for 6 ho...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Short circuit current | aaaaa | aaaaa |
| Open circuit voltage | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com