Triarylamine compounds, preparation method thereof, and application in preparation of solar cells
A compound, triarylamine technology, applied in the field of solar cell materials, can solve the problems of high electron recombination rate, aggregation, low light harvesting ability, etc., and achieve low ionization potential, high hole mobility, strong fluorescence performance and photostability Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
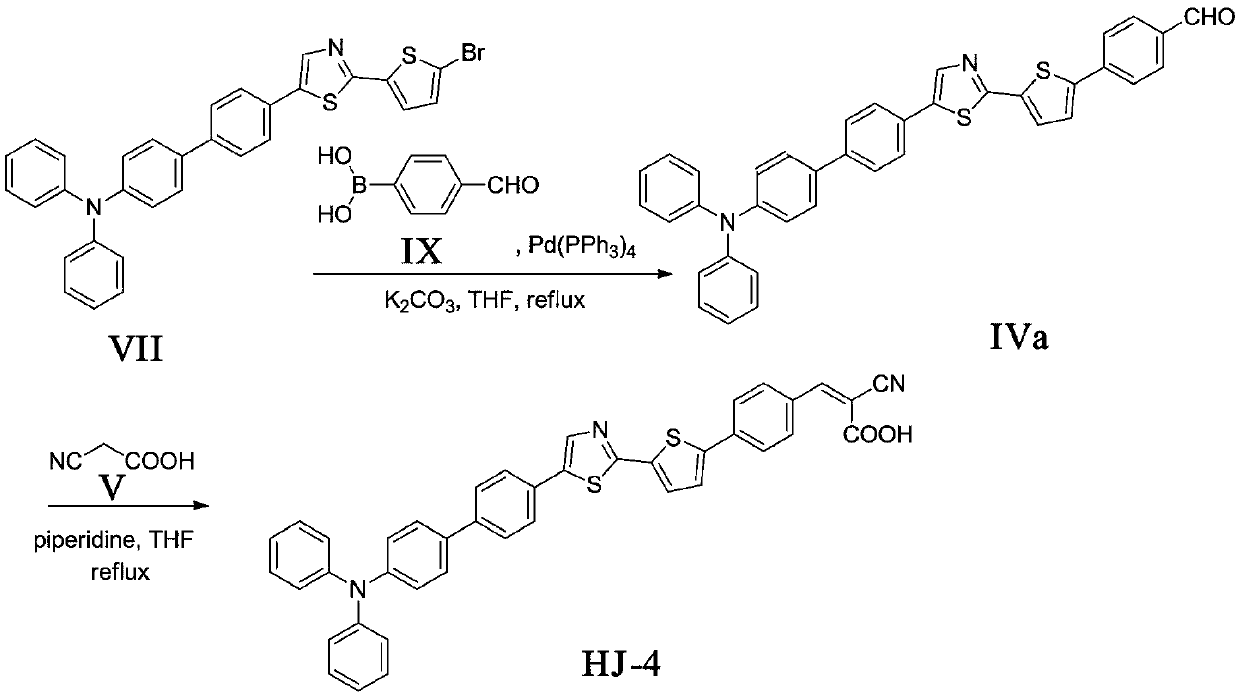
[0031] The synthesis of embodiment 1 compound formula HJ-4
[0032] 1. The synthesis of compound formula IVa
[0033] The compound formula VII (1.13g, 2.0mmol), Pd (PPh 3 ) 4 (0.23g, 0.2mmol), compound formula IX (0.45g, 3.0mmol), potassium carbonate aqueous solution (5mL, 2mol / L) were dissolved in tetrahydrofuran (30mL), N 2 React under protection for about 10 hours. The reaction solution was extracted three times with dichloromethane, the organic layer was washed with water, dried over anhydrous sodium sulfate, the solvent was spin-dried, and the column was separated (V CH2Cl2 :V PE =1:1) to obtain 0.35 g of yellow solid with a yield of 30%. m.p.:210-212℃; 1 H NMR (500MHz, CDCl 3 )δ10.06(s,1H,C H O), 8.04(d, J=8.0Hz, 2H), 7.95(d, J=8.0Hz, 2H), 7.76(d, J=8.3Hz, 2H), 7.68(d, J=8.5Hz, 2H) ,7.61-7.55(m,5H),7.45-7.43(m,2H),7.34(t,J=7.5Hz,2H),7.25-7.21(m,6H),7.14-7.11(m,2H); HREIMS m / z 591.1570[M+H] + , cacld C 38 h 26 N 2 OS 2 for:590.1487.
[0034] 2. Synthesis o...
Embodiment 2
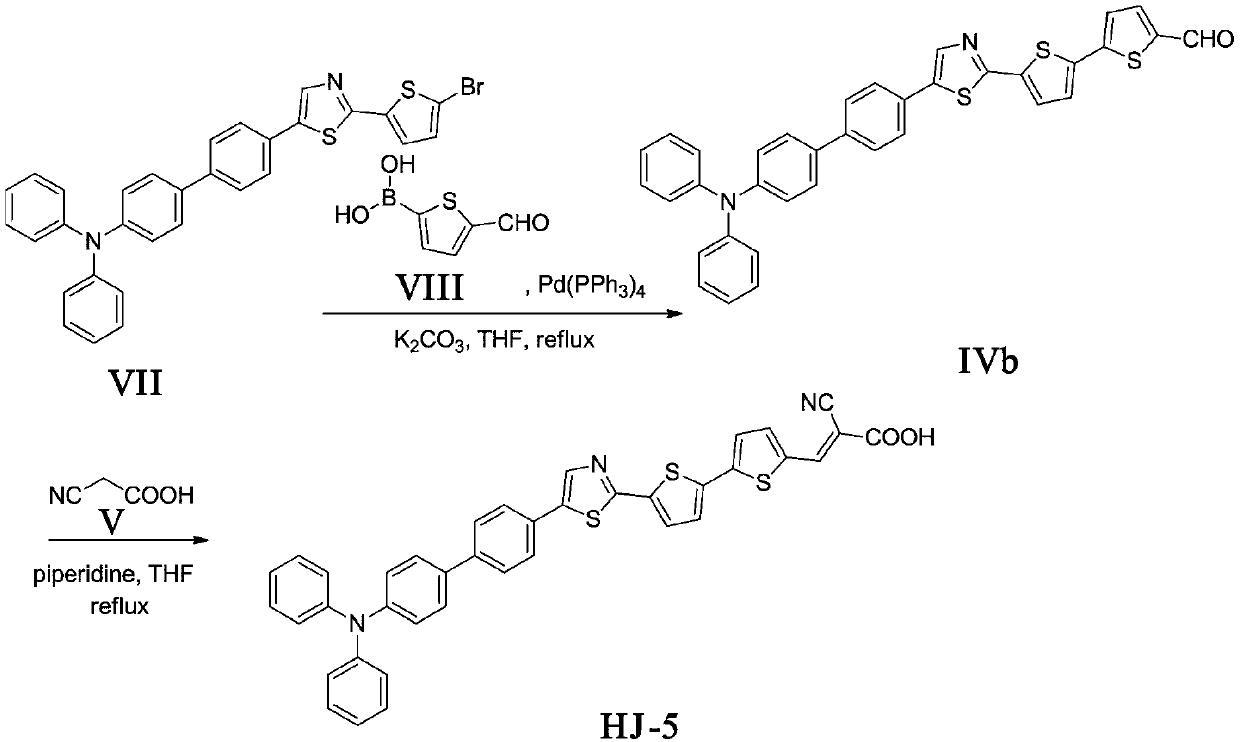
[0036] The synthesis of embodiment 2 compound formula HJ-5
[0037] 1. Synthesis of compound formula IVb
[0038] The compound formula VII (1.13g, 2.0mmol), Pd (PPh 3 ) 4 (0.23g, 0.2mmol), compound formula VIII (0.47g, 3.0mmol), potassium carbonate aqueous solution (5mL, 2mol / L) was dissolved in tetrahydrofuran (30mL), N 2 React under protection for about 10 hours. The reaction solution was extracted three times with dichloromethane, the organic layer was washed with water, dried over anhydrous sodium sulfate, the solvent was spin-dried, and the column was separated (V CH2Cl2 :V PE =1:1) to obtain 0.25 g of orange solid with a yield of 21%. m.p.:117-119℃; 1 H NMR (500MHz, CDCl 3 )δ9.88(s,1H,C HO), 8.04(d, J=8.3Hz, 2H), 7.74(d, J=3.9Hz, 1H), 7.68(d, J=8.3Hz, 2H), 7.63-7.55(m, 5H), 7.44( d,J=6.5Hz,2H)7.34(dd,J=8.7,6.2Hz,3H),7.22(t,J=7.7Hz,4H),7.16-7.12(m,4H).HREIMS m / z 597.1110[ M+H] + , cacld C 36 h 24 N 2 OS 3 for:596.1051.
[0039] 2. Synthesis of compound for...
Embodiment 3
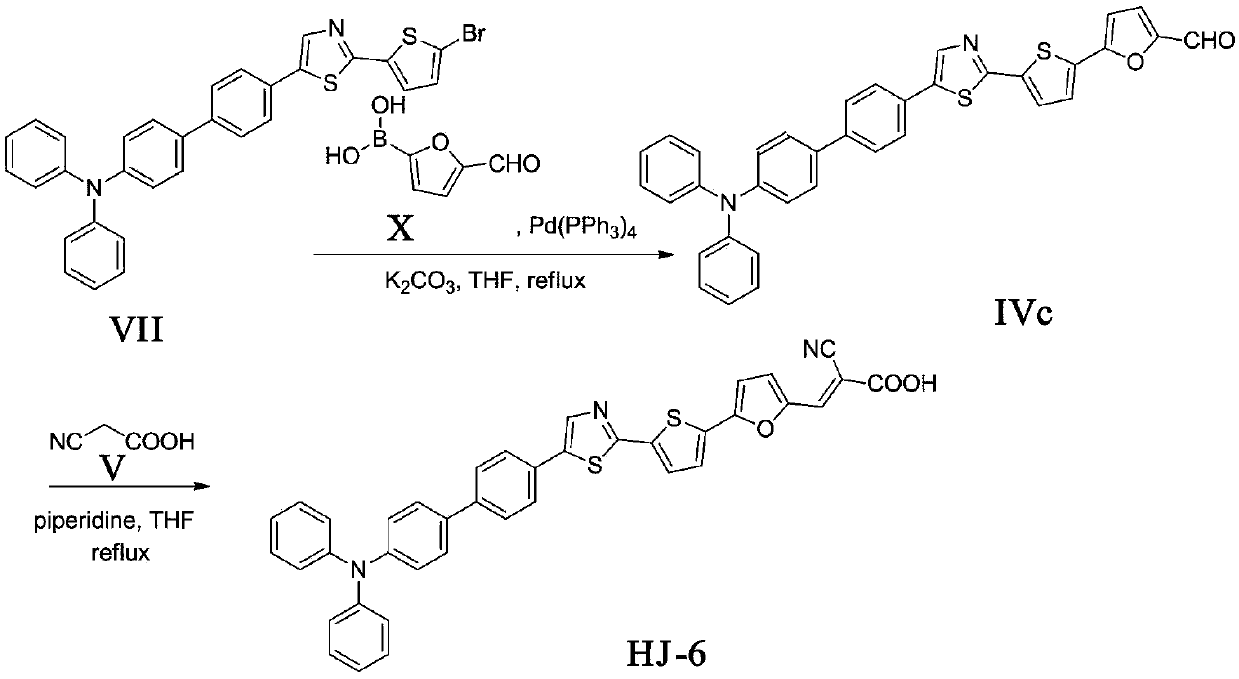
[0041] The synthesis of embodiment 3 compound formula HJ-6
[0042] 1. The synthesis of compound formula IVc
[0043] The compound formula VII (1.13g, 2.0mmol), Pd (PPh 3 ) 4 (0.23g, 0.2mmol), compound formula X (0.42g, 3.0mmol), potassium carbonate aqueous solution (5mL, 2mol / L) was dissolved in tetrahydrofuran (30mL), N 2 React under protection for about 10 hours. The reaction solution was extracted three times with dichloromethane, the organic layer was washed with water, dried over anhydrous sodium sulfate, the solvent was spin-dried, and the column was separated (V CH2Cl2 :V PE =1:1) to obtain 0.46 g of orange solid with a yield of 40%. m.p.:117-119℃; 1 H NMR (500MHz, CDCl 3 )δ9.62(s,1H,C H O), 8.04(d, J=8.4Hz, 2H), 7.71(d, J=8.8Hz, 2H), 7.68(d, J=8.4Hz, 2H), 7.62-7.58(m, 3H), 7.45- 7.43(m,2H),7.35(d,J=8.3Hz,2H),7.33(d,J=3.6Hz,2H),7.23-7.20(m,4H),7.17-7.14(m,3H),7.12 (dd, J=5.0, 3.7Hz, 1H); HREIMS m / z 581.1340[M+H] + , cacldC 36 h 24 N 2 o 2 S 2 for:580.12...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com