New codonopsis pilosula alkynol compound as well as preparation method and application thereof and pharmaceutical composition thereof
A technology of compound and Codonopsis pilosula, which is applied in the field of compound extraction and separation, can solve the problems of complex components of traditional Chinese medicine, unclear active ingredients, and unstable curative effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
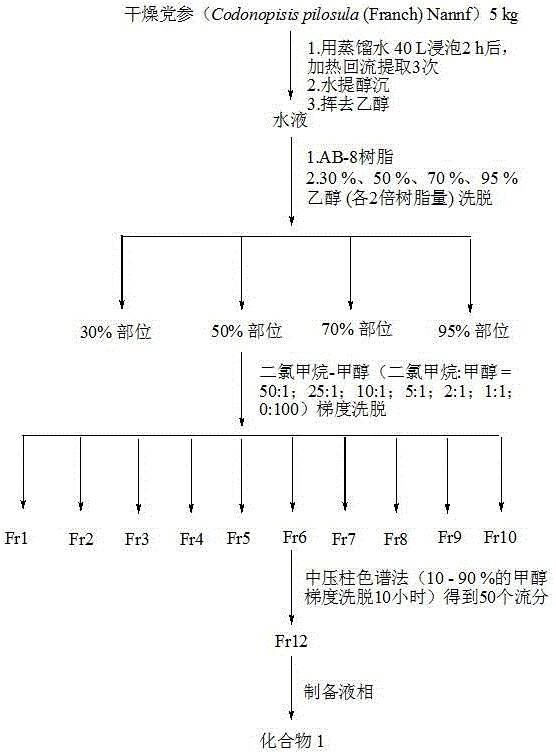
[0027] Example 1: 5 kg of dried Codonopsis pilosula, crushed, soaked in 40 L of distilled water for 2 hours, heated and refluxed for 3 times, 2 hours each time; after water extraction and alcohol precipitation, volatilized ethanol to have no alcohol smell, and mixed with AB-8 resin. Adsorption, after washing with pure water, eluting with 30%, 50%, 70%, 95% ethanol (each 2 times the amount of resin), receiving the eluents of each section, and concentrating under reduced pressure to obtain each extract; each extract Concentrated under reduced pressure to obtain 30%, 50%, 70%, and 95% ethanol elution fractions of 12 g, 60 g, 100 g, and 160 g; the 50 % ethanol elution fractions were subjected to silica gel (200-300 mesh, 500 g) column chromatography , CH 2 Cl 2 -MeOH (100:1→1:1) gradient elution separation gave fractions Fr1-Fr10;. Fr6 (10 g) was separated by medium pressure column chromatography (10 – 90% methanol gradient over 10 hours) In 50 fractions (1 fraction of 500 ml), ...
Embodiment 2
[0028] Example 2: Application of Compound 1 in the preparation of a medicament for preventing and / or treating neurodegenerative diseases. The neurodegenerative diseases are stroke, dementia, neuroinflammation, heavy metal poisoning, and nerve agent poisoning. The dementia is Alzheimer's disease and vascular dementia.
[0029] The physicochemical and spectral data of the new Codonopsis alkynols are as follows:
[0030] Yellow oil, ESI-MS m / z 217 [M+H] + . 1 H-NMR (500 MHz, CD 3 OD) δ : 6.23(1H, dt, J =11.0, 6.0Hz, H-2),6.28(2H, dd, J = 16.0, 5.0Hz, H-9), 5.83(1H, dt, J =15.5, 2.0Hz, H-8), 5.67(1H, dd, J = 11.0, 1.0Hz, H-3), 4.31(2H, dd, J = 6.5,1.5Hz, H-1) ; 13 C-NMR (125 MHz, CD 3 OD) δ : 149.1(C-2), 147.5(C-9), 109.5(C-3), 108.9(C-8), 82.1(C-8), 80.0(C-7), 78.4(C-1') , 77.9(C-6), 74.6(C-5), 69.5(C-5'), 61.3(C-1), 33.0(C-2'), 27.0(C-4'), 24.4(C- 3').
[0031] Pharmacological experiments
[0032]Test material 1. Test drug: the monomer compound of the pres...
experiment example 1
[0033] Experimental Example 1: Influence of the compounds of the present invention on the survival state of neurons in rat cerebral cortex and protective effect in the model of neuronal apoptosis induced by sodium nitroprusside
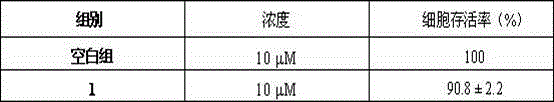
[0034] In the study of the effects of compounds on neuronal survival, primary cultured rat cortical neurons (DIV-9) were divided into control group and administration group (10 μM), n = 6; In the study of the protective effect of the apoptosis model, the primary cultured rat cortical neurons (DIV-7) were divided into control group, sodium nitroprusside (350 μM) modeling group, sodium nitroprusside (350 μM) + Eda Ravone (100 μM) administration group, sodium nitroprusside (350 μM) + compound (10 μM) administration group, n =6. After administration, cells were incubated in a cell incubator for 24 hours, and cell viability was measured by MTT method (570 nm). Taking the absorbance of the control group as the standard, the ratio of the absorbance of each ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com