Method using manganese dioxide to catalyze trifluoromethylation of arenes or heterocyclic arenes
A technology of trifluoromethylation and heterocyclic aromatic hydrocarbons, which is applied in the field of catalytic organic synthesis, can solve the problems of high pollution and high cost, and achieve the effect of simple reaction process, cost reduction and large-scale industrial production
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Example 1 (The substrate is benzene as an example)
[0027] (1) Weigh 0.2mmol of manganese dioxide as the catalyst and 0.5mmol of sodium trifluoromethanesulfinate as the source of trifluoromethyl. After mixing in the reactor, add 1mL of acetonitrile as solvent and 0.5mL of reactant benzene, and seal Parafilm
[0028] (2) After placing the reactor in an ultrasonic cleaning machine with 50KHz ultrasonic treatment for 30s, connect the reactor to an air balloon and place it in a heat-collecting thermostatic magnetic stirrer at 50°C for 24h;
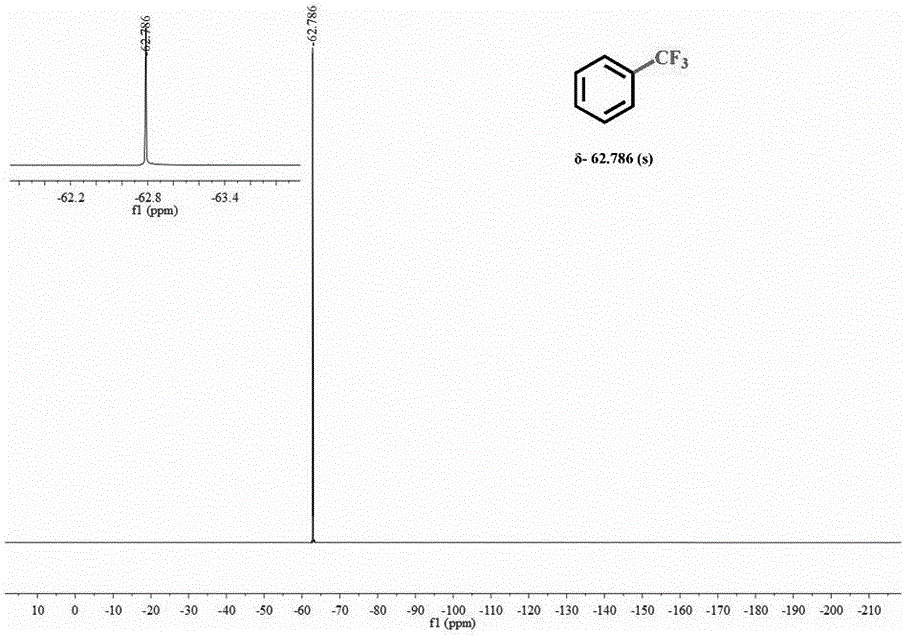
[0029] (3) Centrifuge after the reaction is complete, and pass the supernatant through the column to obtain pure trifluorotoluene and then measure 19 F NMR spectrum (see figure 1 ).
Embodiment 2
[0030] Example 2 (The substrate is benzene as an example)
[0031] (1) Weigh 0.2mmol of manganese dioxide as the catalyst and 0.5mmol of sodium trifluoromethanesulfinate as the source of trifluoromethyl. After mixing in the reactor, add 1mL of acetonitrile as the solvent and 0.5mL of benzene, and seal with the sealing film ;
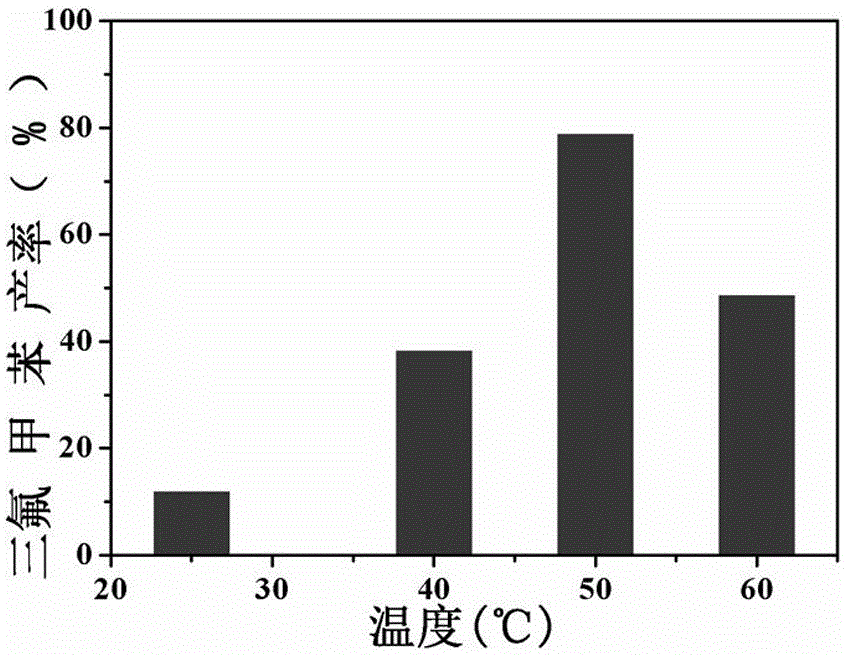
[0032] (2) Put the reactor in an ultrasonic cleaning machine after 50KHz ultrasonic treatment for 30s, connect the reactor to an air balloon, and place it in a heat-collecting thermostatic magnetic stirrer for 24 hours at 25℃, 40℃, and 50℃. ,60℃;
[0033] (3) Centrifuge after the reaction is complete, and detect the supernatant by GC (product volume has been calibrated);
[0034] The result is displayed (see figure 2 ), as the reaction temperature increases, the yield increases gradually, but not infinitely, reaching the highest at 50°C.
Embodiment 3
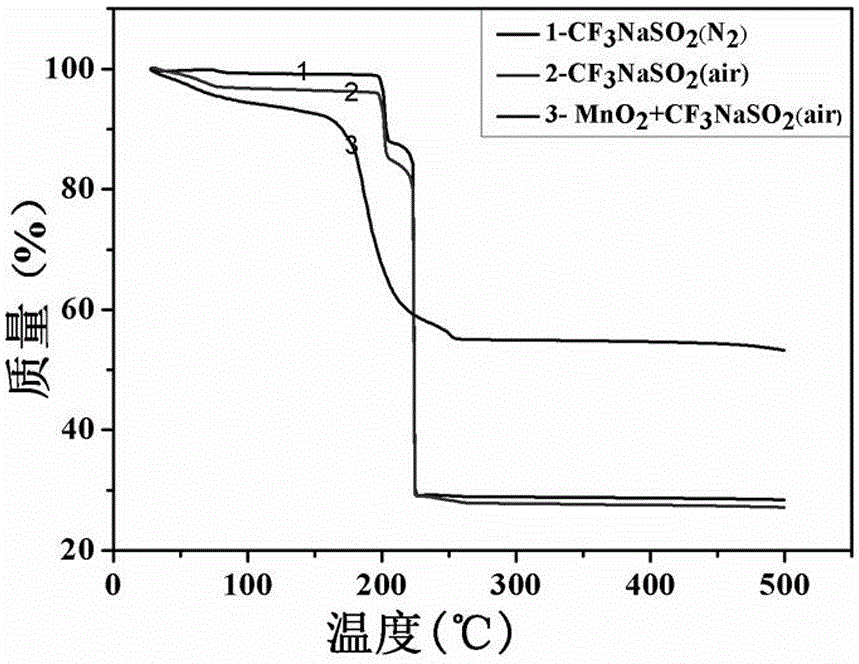
[0036] (1) Weigh 10-20mg sodium trifluoromethanesulfinate as 1, 2 respectively. The thermogravimetric test results are shown in air and nitrogen atmosphere image 3 Curve in 1,2;
[0037] (2) Weigh a mixture of 10 mg of sodium trifluoromethanesulfinate and 10 mg of manganese dioxide for thermogravimetric test under air atmosphere, the results are shown in image 3 Curve 3 in
[0038] (3) The test results show that the decomposition temperature of trifluoromethyl source sodium trifluoromethanesulfinate is 150-190℃, which is much higher than the required temperature of 50℃ for the reaction.
PUM
| Property | Measurement | Unit |
|---|---|---|
| decomposition temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com