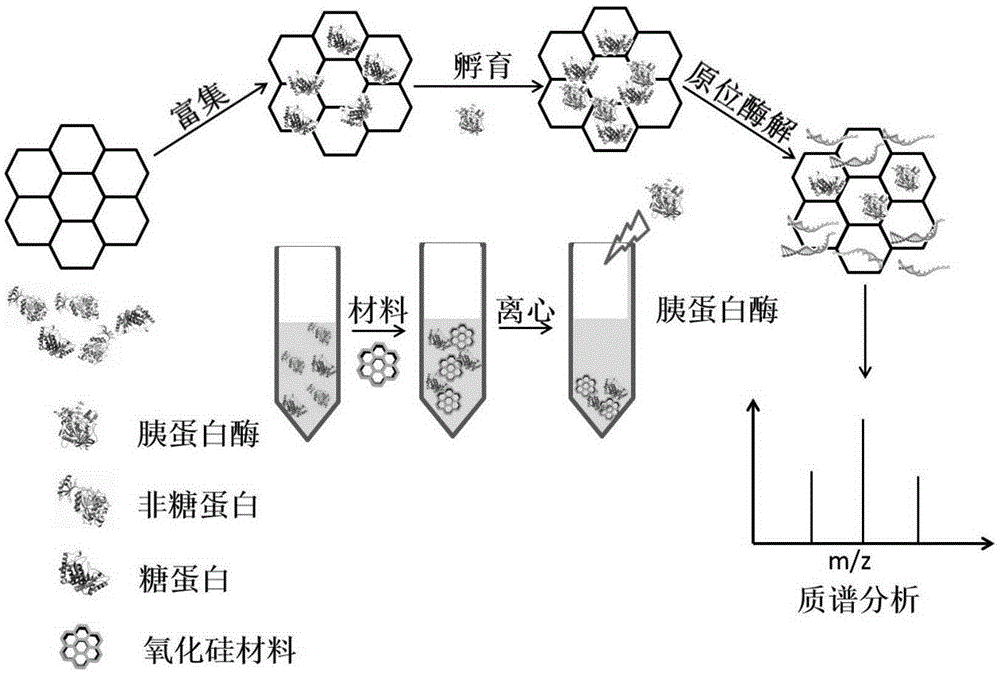
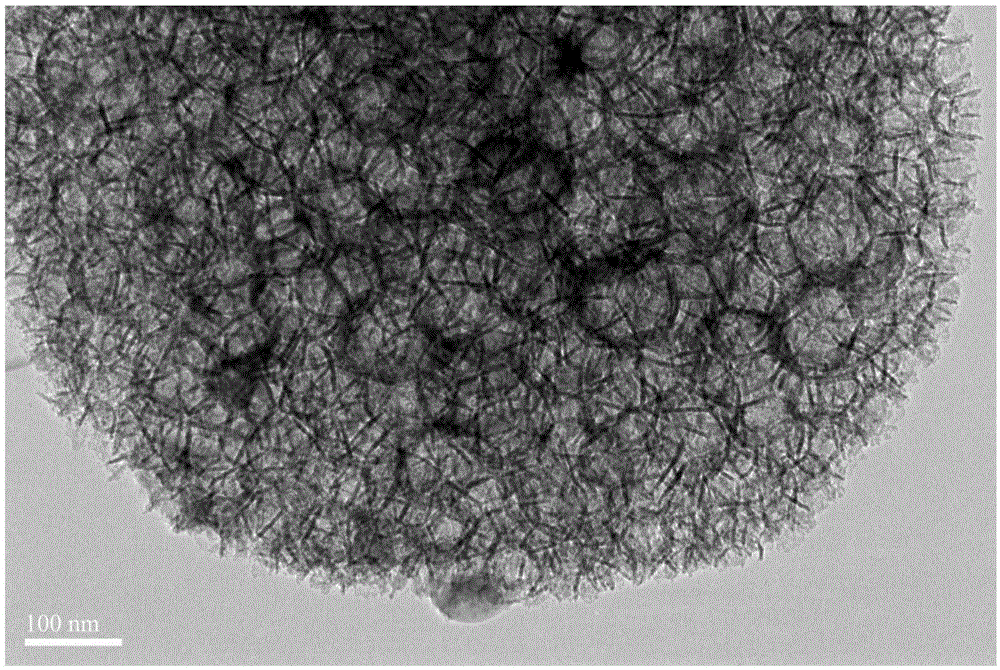
System and method for solid phase enrichment coupled mass spectrometric detection of glycoprotein
A glycoprotein and mass-coupling technology, applied in the field of biochemical analysis, can solve problems such as time-consuming, low ratio, and difficult identification by mass spectrometry
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Example 1: Test of the ability of the hydrazide-modified macroporous silica nanomaterial to enrich cell lysate.
[0030] A cell lysate solution (dissolved in an aqueous acetonitrile solution) is prepared, the material is incubated in the solution for 15-30 minutes, and the material is separated from the solution under centrifugation. Discard the supernatant and add trypsin for quick in situ enzymatic digestion. After freeze-drying the enzymatic hydrolyzate, add trifluoroacetic acid in acetonitrile aqueous solution, and enter the electrospray mass spectrometer for analysis. Hundreds of proteins can be analyzed and detected at one time, and the enrichment efficiency is very high. Some glycoproteins specifically identified are shown in Table 1 .
[0031]
[0032] Table 1. Part of the glycoproteins detected after the enrichment experiment of glycoproteins in cell lysates.
Embodiment 2
[0033] Example 2: Test of hydrazide-modified porous silica nanomaterials for the ability to enrich glycoproteins in serum solutions.
[0034] A serum solution (dissolved in aqueous acetonitrile) was prepared, the material was incubated in the solution for 15-30 minutes, and the material was separated from the solution by centrifugation. Discard the supernatant and add trypsin for quick in situ enzymatic digestion. After freeze-drying the enzymatic hydrolyzate, add trifluoroacetic acid in acetonitrile aqueous solution, and enter the electrospray mass spectrometer for analysis. Hundreds of proteins can be analyzed and detected at one time, and the enrichment efficiency is very high. Some glycoproteins specifically identified are shown in Table 2 .
[0035]
PUM
| Property | Measurement | Unit |
|---|---|---|
| pore size | aaaaa | aaaaa |
| contact angle | aaaaa | aaaaa |
| pore size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com