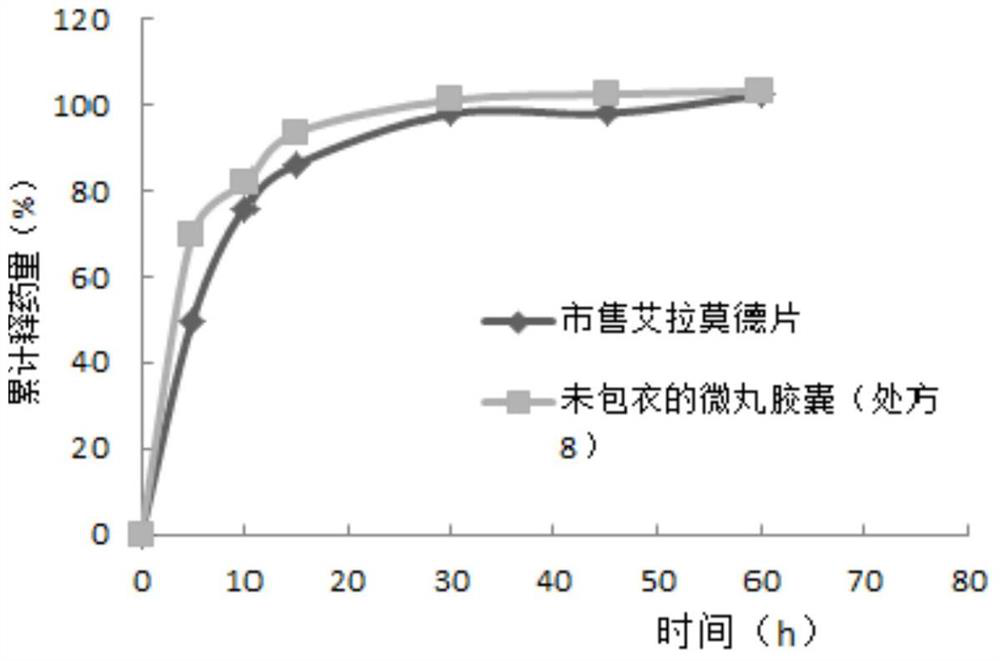
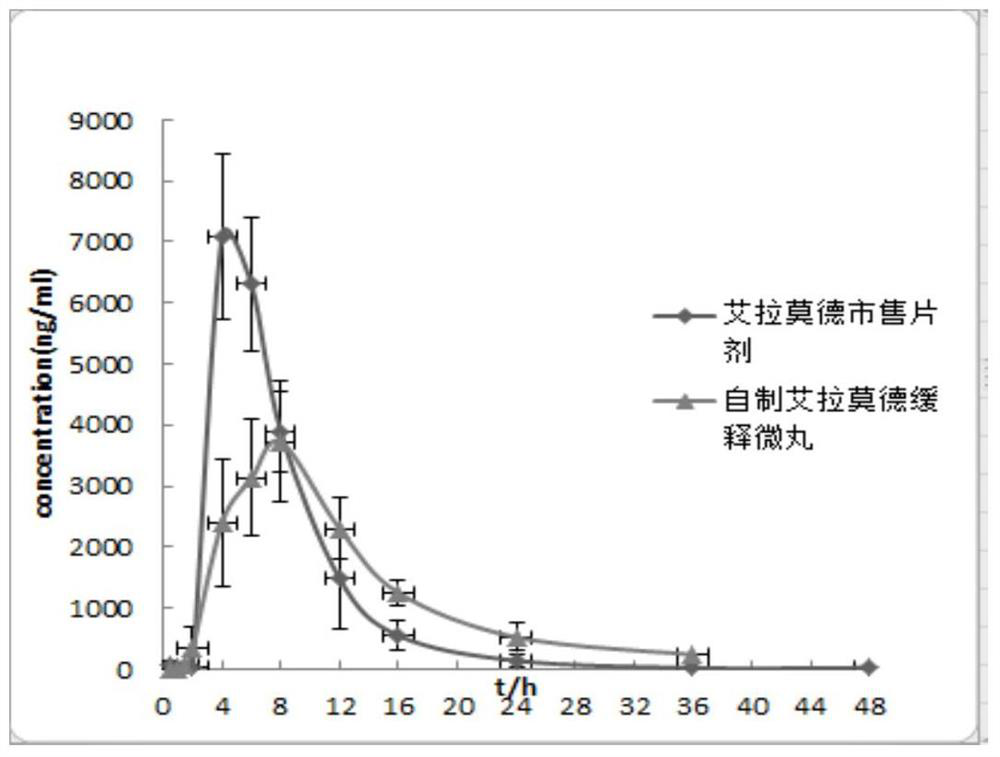
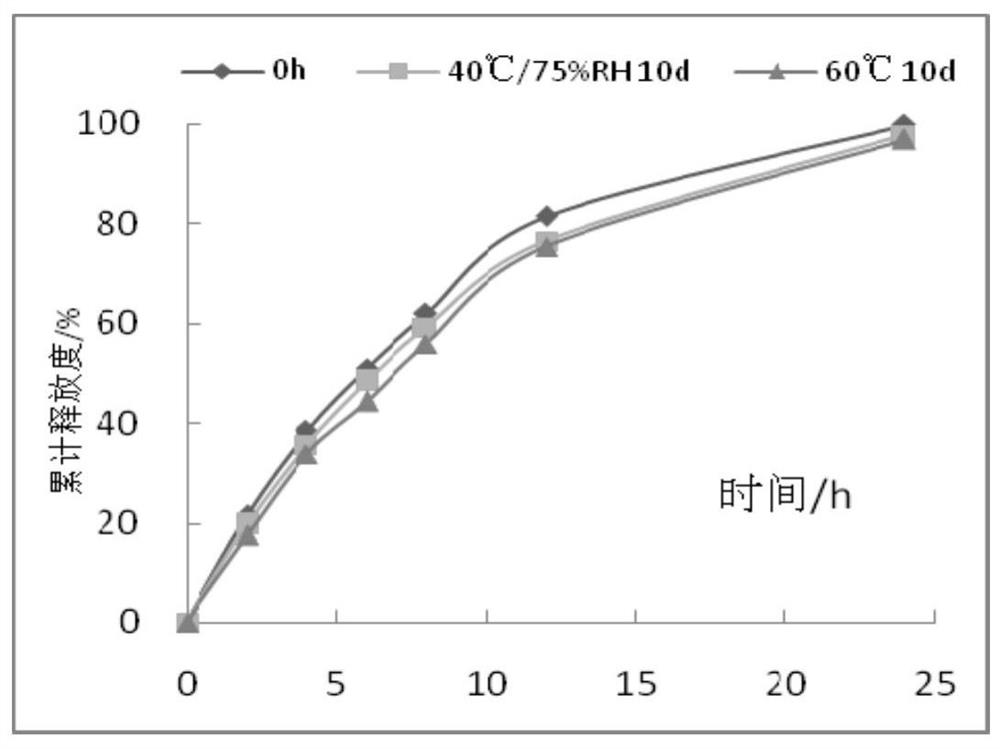
Iguratimod sustained-release capsules and preparation method thereof
A technology of capsules and sustained-release pellets, applied in the field of pharmaceutical preparations, can solve the problems of large fluctuation of blood drug concentration, frequent administration, peak-to-valley phenomenon, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] Embodiment 1 A kind of iguratimod sustained-release capsule and preparation method thereof
[0036] iguratimod extended-release capsules, including extended-release pellets.
[0037] Composition of sustained-release pellets, unit (g):
[0038]
[0039] Preparation Process
[0040] Preparation of the active drug layer: Mix and grind the prescribed amount of iguratimod and microcrystalline cellulose through a 100 sieve for three times, add povidone k30 into water and stir to obtain a 5% binder solution. Add the microcrystalline cellulose core (24-30 mesh) into the coating granulator, the speed of the spray pump starts at 6rpm, wetting for 2-3 minutes, the speed of the main machine is 180rpm, the spray pressure is 0.15Mpa, and the blast volume is 6L / min, the powder supply speed is 5rpm, adjust the speed of the spray pump and the powder supply speed according to the motion state of the pellets and the powder supply state, collect the 16-20 mesh pellets, and dry them a...
Embodiment 2
[0043] Embodiment 2 A kind of iguratimod sustained-release capsule and preparation method thereof
[0044] iguratimod extended-release capsules, including extended-release pellets.
[0045] Composition of sustained-release pellets, unit (g):
[0046]
[0047] Preparation Process
[0048] Preparation of the active drug layer: the prescribed amount of iguratimod, microcrystalline cellulose, and lactose were mixed and ground through a 100 sieve for three times, and povidone k30 was added to water to obtain a 5% binder solution. Add 400g of sucrose ball cores (25-30 mesh) into the coating granulator, the speed of the spray pump is 5rpm at the beginning, wetting for 5min, the speed of the main machine is 200rpm, the spray pressure is 0.2Mpa, and the blast volume is 8L / min. The powder supply speed is 7rpm. Adjust the speed of the spray pump and the powder supply speed according to the movement state of the pellets, collect the pellets of 16-20 meshes, and dry them at 50°C for 4...
Embodiment 3
[0051] Embodiment 3 A kind of iguratimod sustained-release capsule and preparation method thereof
[0052] iguratimod extended-release capsules, including extended-release pellets.
[0053] Composition of sustained-release pellets, unit (g):
[0054]
[0055] Preparation Process
[0056] Preparation of the active drug layer: the prescribed amount of iguratimod, powdered microcrystalline cellulose and lactose were mixed and ground through a 100 sieve for three times, and povidone k30 was added to water to obtain a 6% binder solution. Add 420g of sucrose ball cores (25-30 mesh) into the coating granulator, the speed of the spray pump is 8rpm at the beginning, wetting for 5min, the speed of the main machine is 220rpm, the spray pressure is 0.2Mpa, and the blast volume is 10L / min. The powder supply speed is 8rpm, adjust the speed of the spray pump and the powder supply speed according to the movement state of the pellets, collect the 16-20 mesh pellets, and dry them at 50°C f...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com