Compositions
An aqueous solution and solution technology, applied in the direction of drug combination, drug delivery, inorganic non-active ingredients, etc., can solve the problem of no chromatography
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
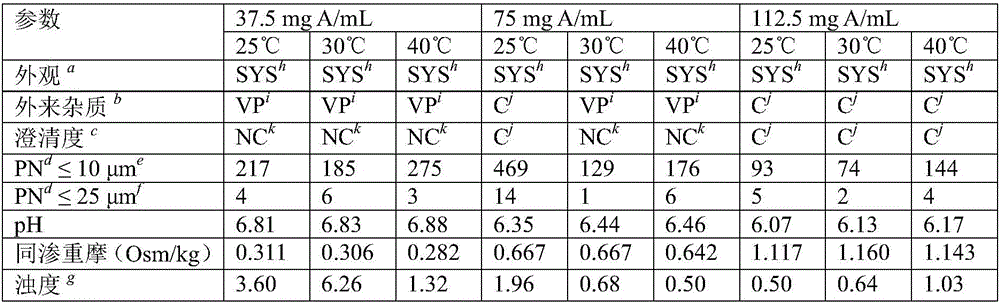
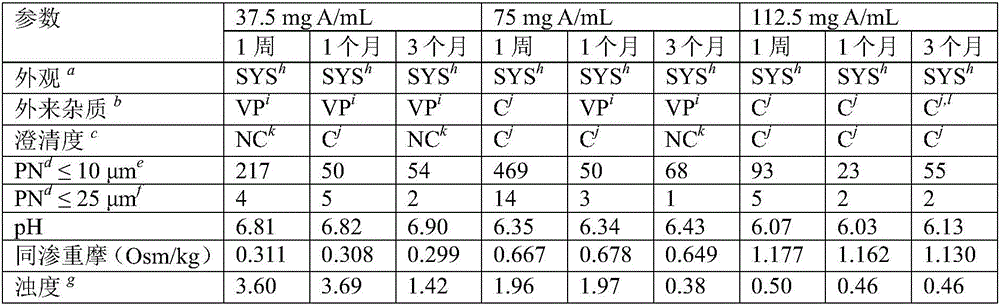
[0094] Aqueous solutions containing colistin sodium methanesulfonate (CMS) at three different concentrations (37.5 mg A / mL, 75 mg A / mL and 112.5 mg A / mL) were stored in airtight containers for a period of one week.
[0095] HPLC chromatograms (data not shown) showed degradation in aqueous solutions containing 37.5 mg A / mL of CMS, but not significant degradation in aqueous solutions containing 75 mg A / mL of CMS or 112.5 mg A / mL of CMS.
[0096] At temperatures (5°C (data not shown), 25°C, 30°C, and 40°C), several parameters of the above composition were observed for the stated time, including but not limited to: appearance, foreign matter (USP, the presence of visible particles ("VP") is a non-compliance), the clarity of the solution (USP, lack of clarity leads to non-compliance), the number of microscopically visible particles (≤10 μm and ≤25 μm two Those, USP), pH (USP), osmolality (USP), and turbidity (Ph.Eur.2.2.1). The results of these observations are presented in Table 2...
Embodiment 2
[0110] Aqueous solutions containing 94 mg A / mL colistin sodium methanesulfonate (CMS) were stored in airtight containers at different temperatures (5°C, 25°C, 30°C and 40°C) for 1 month. HPLC chromatograms (data not shown) showed no significant changes.
[0111] Several parameters of the composition described above were observed over a period of 1 month and the results are summarized in Table 4.
[0112] Table 4.
[0113] parameter initial 5℃ 25℃ 30℃ 40℃ Exterior a
SYS h
SYS h
SYS h
SYS h
SYS h
C j
C j
C j
C j
C j
Clarity c
C j
C j
C j
C j
C j
PN d ≤10μm e
102 11 34 33 59 PN d ≤25μm f
2 0 2 3 1 pH 6.21 5.98 6.09 6.11 6.13 Osmolality (Osm / kg) 0.928 0.916 0.925 0.908 0.929 Turbidity g
0.4 0.3 0.3 0.4 0.4
[0114] Instructions and Notes
[0115] a Colorless to slightly yellow so...
Embodiment 3
[0119] The viscosity values of the compositions comprising CMS at different compositions were measured at 25°C and the results are summarized in Table 5.
[0120] table 5.
[0121] sample Concentration (mg A / ml) Viscosity (cSt) water 0.0 0.94 1 37.5 1.25 2 75.0 1.89 3 94.0 2.24 4 112.5 3.05
[0122] The viscosity (η) values plotted against the concentration (c) values show a quadratic relationship (η=Ac2+Bc+C) with good agreement (R2=0.9901), where A=0.0002, B=0.0003 and C= 0.9642.
[0123] In view of the functional relationship shown above, another aspect of the first and second embodiments is a composition comprising a sulfomethylated colistin having a viscosity value as determined by the quadratic expression above.
[0124] While a full and complete description is believed to be contained herein, certain patent and non-patent documents, including the aforementioned USP and Ph. Eur. monographs, may cover certain major ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com