Liquid-state NAG (glucosaminidase) correction solution and preparation method thereof
A calibration liquid and liquid technology, applied in the field of NAG calibration liquid, can solve the problems of increasing the cost of biochemical diagnostic reagents, harsh storage conditions, and inconvenience for users, and achieve great social and economic benefits, good stability, and simple preparation Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] Configure 1L liquid NAG calibration solution of the present invention, the method is as follows:
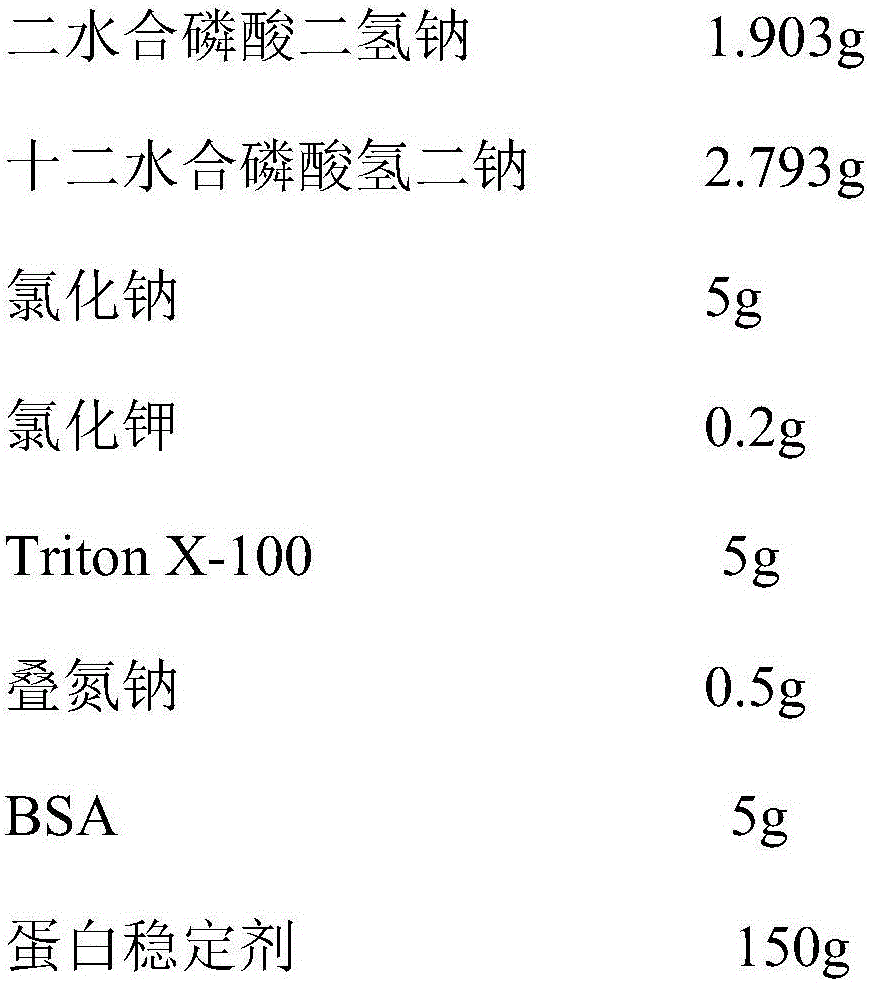
[0028] Weigh each component according to the following formula:
[0029]
[0030] Dissolve the above components in 900mL deionized water, stir and dissolve fully, adjust the pH value to 7.4 to obtain the NAG calibration solution dilution; then add 45Uβ-N-acetylglucosaminidase to the NAG calibration solution dilution, and use Dilute to 1L with deionized water to obtain the NAG calibration solution; filter it through a 0.22μm filter membrane and store it at 2-8°C.
Embodiment 2
[0032] Configure 1L liquid NAG calibration solution of the present invention, the method is as follows:
[0033] Weigh each component according to the following formula:
[0034]
[0035] Dissolve the above components in 900mL deionized water, stir and dissolve fully, adjust the pH value to 6.3 to obtain the NAG calibration solution dilution, then add 45Uβ-N-acetylglucosaminidase to the NAG calibration solution dilution, and use Dilute to 1L with deionized water to obtain the NAG calibration solution; filter it through a 0.22μm filter membrane and store it at 2-8°C.
Embodiment 3
[0037] Configure 1L liquid NAG calibration solution of the present invention, the method is as follows:
[0038] Weigh each component according to the following formula:
[0039]
[0040] Dissolve the above components in 900mL deionized water, stir and dissolve fully, adjust the pH value to 7.6 to obtain the NAG calibration solution dilution, then add 45Uβ-N-acetylglucosaminidase to the NAG calibration solution dilution, and use Dilute to 1L with deionized water to obtain the NAG calibration solution; filter it through a 0.22μm filter membrane and store it at 2-8°C.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com