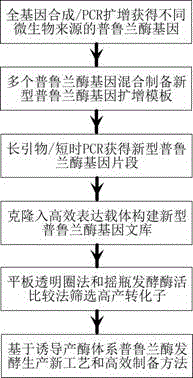
Method for acquiring novel pullulanase gene and high-yield strain and technology for producing enzyme
A technology of pullulanase and gene, which is applied in the field of genetic engineering and fermentation engineering, can solve the problem of high action temperature and achieve the effects of efficient preparation, high catalytic activity and easy acquisition
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0048] Embodiment 1—the synthesis of pullulanase gene from different sources
[0049] According to the accession number information in Table 1, each pullulanase gene sequence was downloaded from the NCBI database and synthesized.
[0050] Table 1 Pullulanase genes and their accession numbers
[0051]
Embodiment 2
[0052] Embodiment 2—Acquisition of novel pullulanase gene library
[0053] The pullulanase gene obtained in Example 1 was used as a template to clone the pullulanase gene obtained in different combinations. Use part of the bases in the relatively conserved sequence of the above-mentioned microbial pullulanase gene to form long primers in series, such as PULX1 and PULX2 in the appendix, under short-term PCR conditions [95°C 10min; 30×(94°C 30s; 56°C 1min; 72°C 0.1~2s); 68°C 10min] to amplify the target band. The obtained bands were cloned into the expression vector pHY-WZX to obtain the recombinant plasmid pHY-WZX-pulX. The obtained recombinant plasmid was preserved in Escherichia coli JM109. As a novel pullulanase gene library, it is used for the construction of subsequent secretory expression recombinant bacteria and the screening of pullulanase high-producing strains.
Embodiment 3
[0054] Embodiment 3—screening of novel pullulanase high-yielding bacterial strains
[0055] The above-mentioned recombinant plasmid was extracted by alkaline lysis to prepare a closed-circle plasmid and introduced into the host bacteria Bacillus licheniformis CBB302 by electric shock transformation method to obtain the corresponding recombinant bacteria; Great strain. The recombinant bacteria obtained from the primary screening were inoculated into the liquid pullulanase screening medium and cultured aerobically at 37 °C for 120 h to measure the enzyme production. The enzyme activity data are shown in Table 2. The strains with obvious transparent circles were stored in frozen storage tubes at -70°C after liquid culture for subsequent fermentation process establishment and efficient preparation of pullulanase.
[0056] Table 2 The formation of the transparent zone of the new pullulanase recombinant bacteria
[0057]
[0058]
[0059]
[0060]
[0061] Note: + / -, t...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Diameter | aaaaa | aaaaa |
| Diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com