6-chloro-2-aminobenzothiazole derivative as well as preparation method and application thereof
An aminobenzene and thiazole technology, which is applied in the field of 6-chloro-2-aminobenzothiazole derivatives, can solve problems such as high toxicity, and achieve the effects of simple preparation method, cheap raw materials and good inhibitory activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
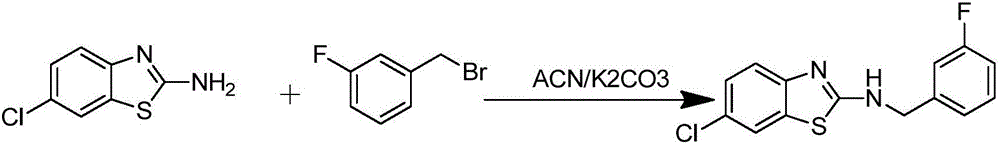
[0037] Example 1 Preparation of 6-chloro-N-(3-fluorobenzyl)benzo[d]thiazol-2-amine (K1):
[0038]
[0039] 3.4 mmol of 2-amino-6-chlorobenzothiazole, 2 mmol of K 2 CO 3 Add it into a 100ml three-neck bottle as an acid-binding agent, then add 6ml of acetonitrile to dissolve it ultrasonically, use a pipette gun to absorb 1mmol of 3-fluorobenzyl bromide as the raw material, dissolve it in 15ml of acetonitrile, and drop it at a rate of 1 drop / 5s with a constant pressure burette. Put it into a three-necked bottle, heat and reflux for 6-7 hours, and monitor the reaction process by TLC. After the reaction, the reaction liquid was lowered to room temperature, and the solvent was evaporated under reduced pressure. Add 8ml of water and an equal volume of ethyl acetate to extract, repeat a small amount of extraction for 3 times, discard the aqueous layer, and use Na 2 SO 4 dry. The dried organic layer was evaporated to dryness under reduced pressure, made sand and passed through ...
Embodiment 2
[0042] Example 2 Preparation of 6-chloro-N-(4-methylbenzyl)benzo[d]thiazol-2-amine (K2)
[0043]
[0044] 3.4 mmol of 2-amino-6-chlorobenzothiazole, 2 mmol of K 2 CO 3 Add it to a 100ml three-neck bottle as an acid-binding agent, then add 6ml of acetonitrile to dissolve it ultrasonically, absorb 1mmol of the raw material 4-methylbenzyl bromide with a pipette gun, dissolve it in 15ml of acetonitrile, and use a constant pressure burette at a rate of 1 drop / 5s. Drop it into a three-necked bottle at a high speed, heat to reflux for 6-7 hours, and monitor the reaction process by TLC. After the reaction, the reaction liquid was lowered to room temperature, and the solvent was evaporated under reduced pressure. Add 8ml of water and an equal volume of ethyl acetate to extract, repeat a small amount of extraction for 3 times, discard the aqueous layer, and use Na 2 SO 4 dry. The dried organic layer was evaporated to dryness under reduced pressure, made sand and passed through t...
Embodiment 3
[0047]Example 3 Preparation of 6-chloro-N-(4-nitrobenzyl)benzo[d]thiazol-2-amine (K3)
[0048]
[0049] 3.4 mmol of 2-amino-6-chlorobenzothiazole, 2 mmol of K 2 CO 3 Add it into a 100ml three-necked bottle as an acid-binding agent, then add 6ml of acetonitrile for ultrasonic dissolution, weigh 1mmol of the raw material 4-nitrobenzyl bromide and dissolve it in 15ml of acetonitrile, and drop it into three In the flask, it was heated to reflux for 6-7 hours, and the reaction process was monitored by TLC. After the reaction was over, the reaction solution was lowered to room temperature, and the solvent was evaporated to dryness under reduced pressure. Add 8ml of water and an equal volume of ethyl acetate for extraction, insoluble matter appears, filter with suction, discard the solid, wash the solid with a small amount of water and ethyl acetate, repeat the extraction for 3 times, discard the aqueous layer, and wash the organic layer with Na 2 SO 4 dry. The dried organic ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com