Modified ZIFs (Zeolitic Imidazolate Frameworks) material with high CO2 adsorption property and preparation method of modified ZIFs material
An adsorption performance, CO2 technology, applied in separation methods, chemical instruments and methods, other chemical processes, etc., can solve the problems of limiting the effective diffusion of molecules, high pressure at the adsorption opening, etc., achieve excellent adsorption performance, improve adsorption performance, and improve diffusion The effect of restriction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] Weigh 20.4 g (300 mmol) of imidazole and 12.0 g (300 mmol) of NaOH, add them into 20 mL of DMSO, protect with nitrogen, heat to 65°C under stirring, and reflux for 4 hours. Then slowly add 1,6-dibromohexane (100 mmol), after the reaction, remove the solvent DMSO by rotary evaporation, extract 3 times with chloroform, then wash 3 times with saturated brine, wash 3 times with water, concentrate by rotary evaporation, Dry in vacuo to give 1,6-bis(imidazol-1-yl)hexane.
[0021] Weigh 25mmol 2-methylimidazole and 1,6-bis(imidazol-1-yl)hexane (wherein, 2-methylimidazole and 1,6-bis(imidazol-1-yl)hexane molar ratio is 2 : 1), dissolved in 62.5 mL of anhydrous methanol, and then the above solution was placed in a hydrothermal synthesis kettle and kept at 50 °C for 2 h. Weigh 1.89 g of zinc nitrate hexahydrate and dissolve it in 62.5 mL of anhydrous methanol. The cooled former was slowly added dropwise to methanol solution of zinc nitrate, and vigorously stirred at room temper...
Embodiment 2-5
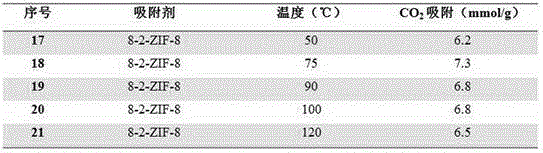
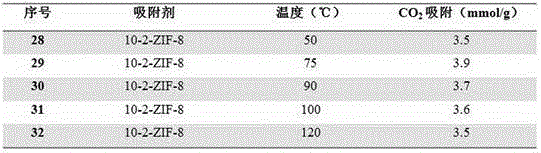
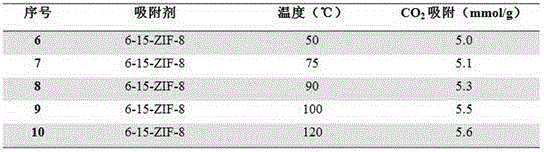
[0023] Same as Example 1, change 2-methylimidazole and 1,6-bis(imidazol-1-yl) hexane molar ratio x, obtain a series of ZIFs materials, marked as 6-x-ZIF-8, wherein x is respectively 5, 10, 15, 20.
Embodiment 6
[0025] Weigh 20.4 g (300 mmol) of imidazole and 12.0 g (300 mmol) of NaOH, add them into 20 mL of DMSO, protect with nitrogen, heat to 65°C under stirring, and reflux for 4 hours. Then slowly add 1,8-dibromooctane (100 mmol). After the reaction, the solvent DMSO was removed by rotary evaporation, extracted three times with chloroform, then washed three times with saturated brine and three times with water, and concentrated by rotary evaporation. Drying in vacuo gave 1,8-bis(imidazol-1-yl)octane.
[0026] Weigh 25mmol 2-methylimidazole and 1,8-bis(imidazol-1-yl) octane (wherein, 2-methylimidazole and 1,8-bis(imidazol-1-yl) octane molar ratio is 1 : 1) Dissolve in 62.5 mL of anhydrous methanol, and then place the above solution in a hydrothermal synthesis kettle and keep at 50°C for 2 h. Weigh 1.89 g of zinc nitrate hexahydrate and dissolve it in 62.5 mL of anhydrous methanol. The former was slowly added dropwise to methanol solution of zinc nitrate, and vigorously stirred at ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Adsorption capacity | aaaaa | aaaaa |
| Adsorption capacity | aaaaa | aaaaa |
| Adsorption capacity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com