Modulators of toll-like receptors for the treatment of hiv
A treatment plan, pharmaceutical technology, applied in antiviral agents, antibody medical ingredients, resistance to vector-borne diseases, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
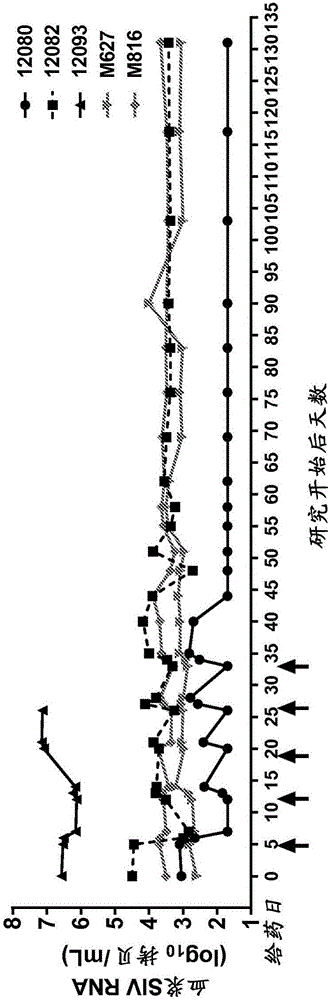
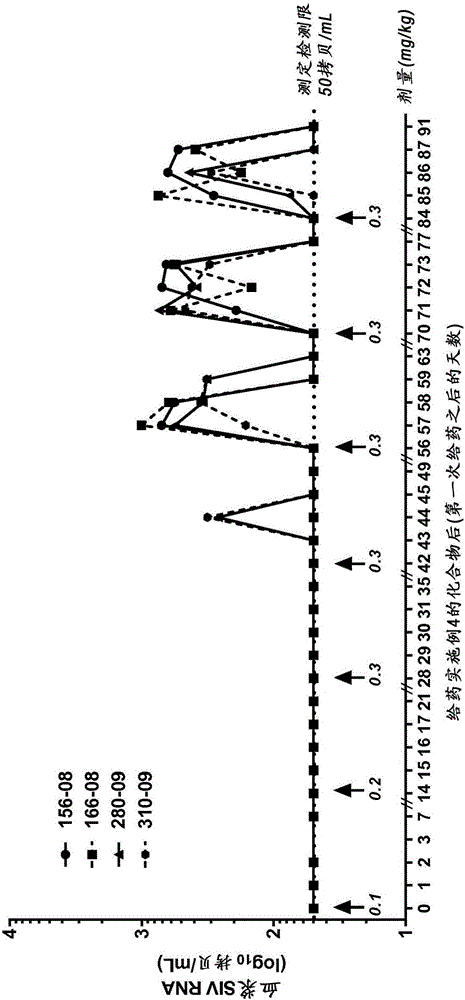
Image
Examples
Embodiment 119
[0396] Example 119: 6-Amino-2-butoxy-9-(3-(pyrrolidin-1-ylmethyl)benzyl)-9H-purin-8-ol
[0397]
[0398] The compound of Example 119 can be prepared using the method of US Patent No. 7,968,544 (Graupe et al.), where it appears as Compound W.
Embodiment 120
[0399] Example 120: 4-Amino-6-(2-methoxyethoxy)-1-((4'-(pyrrolidin-1-ylmethyl)-[1,1'-biphenyl]- 4-yl)methyl)-1H-imidazo[4,5-c]pyridin-2(3H)-one
[0400]
[0401] The compound of Example 120 can be prepared using the methods of WO 2011 / 049825 and U.S. 8,507,507 (Halcomb et al.), where it appears as Compound AX.
Embodiment 121
[0402]Example 121: 4-Amino-7-chloro-6-(2-methoxyethoxy)-1-((4'-(pyrrolidin-1-ylmethyl)-[1,1'-bi Phenyl]-4-yl)methyl)-1H-imidazo[4,5-c]pyridin-2(3H)-one
[0403]
[0404] The compound of Example 121 can be prepared using the methods of WO 2011 / 049825 and U.S. 8,507,507 (Halcomb et al.), where it appears as compound AY.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com