Synthetic method of β-lactamase inhibitor avibactam
A technology of β-lactamase and synthesis method, which is applied in the field of preparation of β-lactamase inhibitors, can solve the problems of cumbersome route process, low final yield, and difficult operation, and achieve stable process, few by-products, and post-processing Handle simple effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
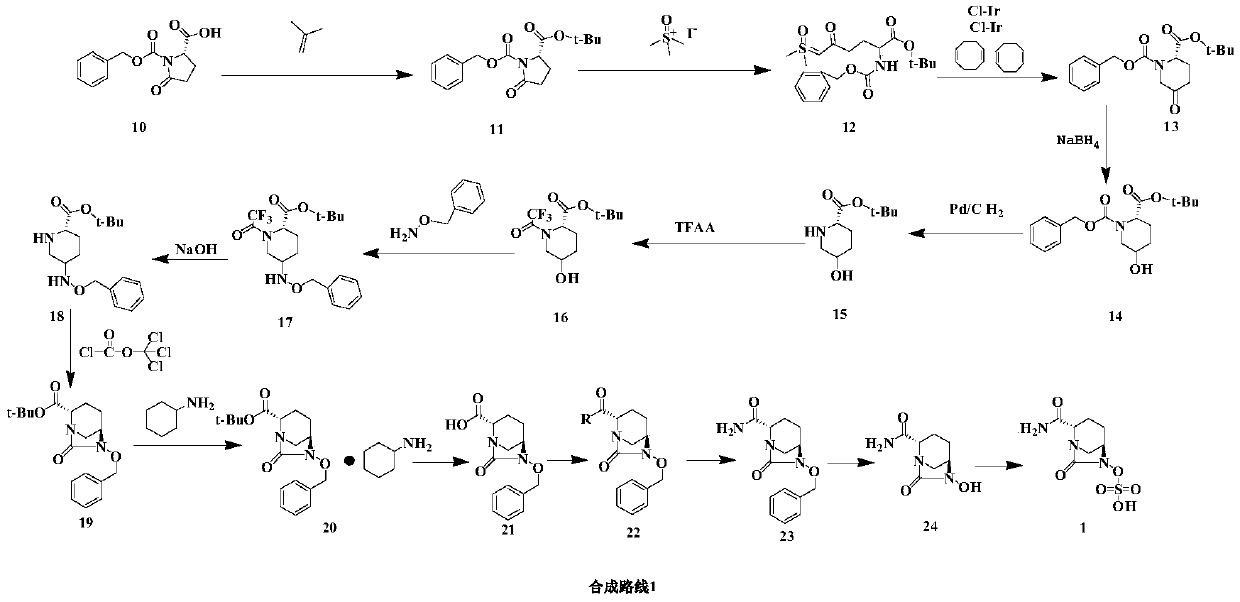
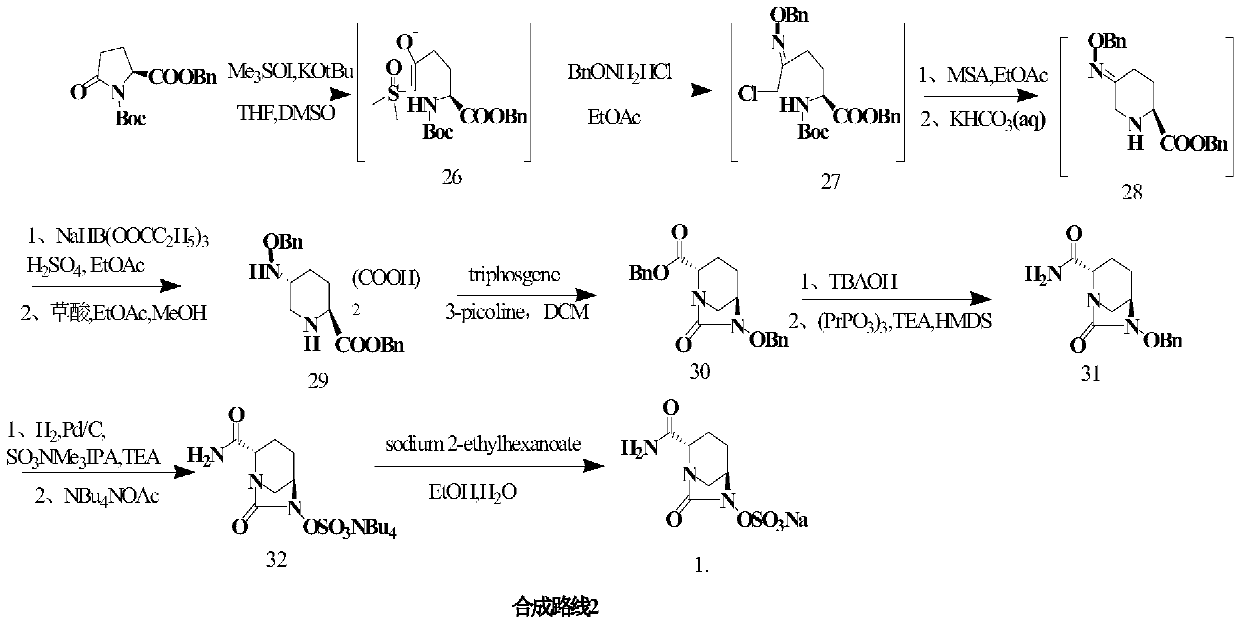
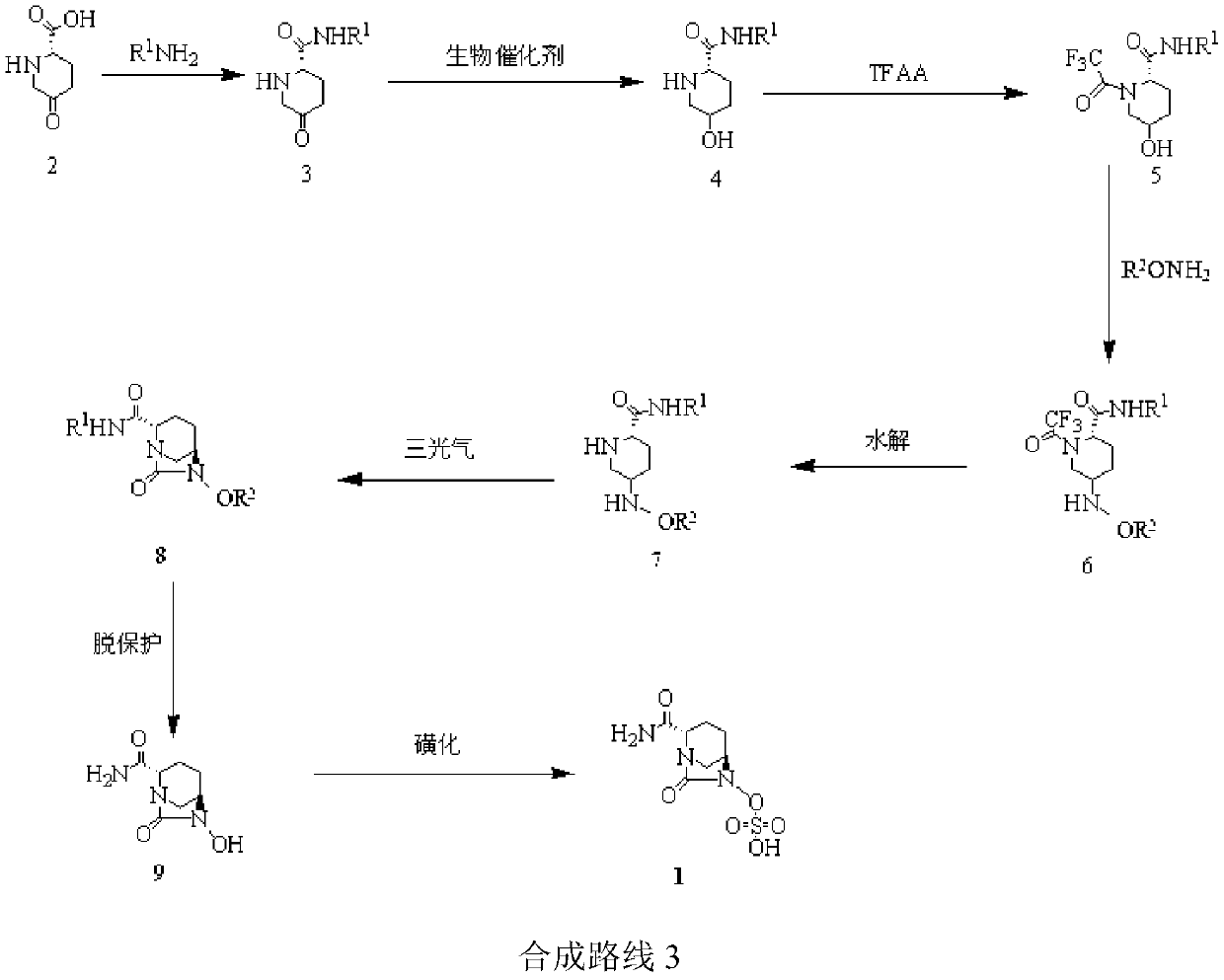
Image
Examples
Embodiment 1
[0032] (1) compound Synthesis:
[0033] Add (S)-5-oxo-2 piperidinecarboxylic acid (25.0g, 174.6mmol) and dichloromethane (300ml) into the three-necked flask and stir, add 2,6-lutidine (1g, 9mmol), Benzylamine hydrochloride (26.5g, 184.8mmol), warmed up and refluxed for 2h, washed with purified water and saturated sodium chloride solution, separated, concentrated to give light brown oily compound 3 (42.83g, 171.1mmol, yield 98% )
[0034] (2) compound Synthesis:
[0035] Add purified water (600ml), glucose (20g), citric acid (0.5g), PEG 2000 (0.1g), disodium hydrogen phosphate (0.5g), dry baker’s yeast (40g) into a three-necked flask, and stir at 30°C 1h, add compound 3 (40g, 172.2mmol) in ethyl acetate solution (300ml) in three batches, each batch was separated by 1h, after the addition was completed, it was kept at 30°C for 24h, filtered, separated, and the aqueous phase was extracted with ethyl acetate , the organic phases were combined, concentrated, crystallized wit...
Embodiment 2
[0049] Others are the same as in Example 1, the difference is:
[0050] (1) In the synthesis of compounds , compound 2 and R 1 NH 2 The reaction produces compound 3, R 1 It is Teoc; temperature is raised to reflux for 1h (yield 96%).
[0051] (2) in the synthesis of compounds During the process, the glucose was replaced by sucrose; the organic solvent ethyl acetate was replaced by acetone; after adding the raw materials, the reaction was incubated at 5°C for 72h (yield 94%, optical activity ee value 99%).
[0052] (3) In the synthesis of compounds At this time, trifluoroacetic anhydride was added dropwise to react for 0.5 h, and the temperature was maintained at -20°C (yield 96%).
[0053] (4) in the synthesis of compounds , after adding benzyloxyamine, maintain the temperature for 1h, wherein R 1 for Teoc, R 2 To Alloc (yield 96%).
[0054] (5) in the synthesis of compounds when (where R 1 for Teoc, R 2 For Alloc), the potassium carbonate solution was replac...
Embodiment 3
[0059] Others are the same as in Example 1, the difference is:
[0060] (1) In the synthesis of compounds , compound 2 and R 1 NH 2 The reaction produces compound 3, R 1 It is Dmb; temperature rises and refluxes for 20h (yield 97%).
[0061] (2) in the synthesis of compounds During the process, the glucose was replaced with sucrose; the organic solvent ethyl acetate was replaced with dichloromethane; after the raw materials were added, the reaction was incubated at 40° C. for 1 h (yield 95%, optical activity ee value 98%).
[0062] (3) In the synthesis of compounds , and reacted for 12 hours after adding trifluoroacetic anhydride dropwise, the temperature was maintained at 0°C (yield 96%).
[0063] (4) in the synthesis of compounds , after adding benzyloxyamine, maintain the temperature for 10h, wherein R 1 for Teoc, R 2 It is Fmoc (yield 96%).
[0064] (5) in the synthesis of compounds when (where R 1 for Teoc, R 2 For Fmoc), the potassium carbonate solution...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com