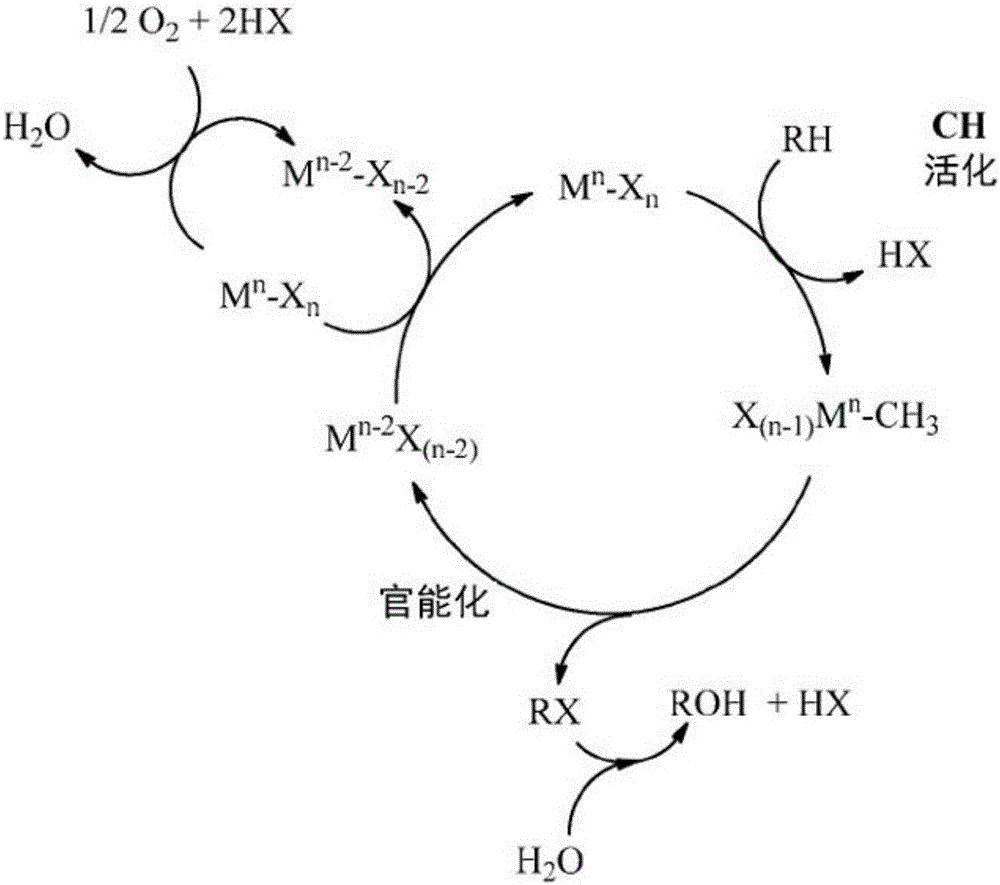
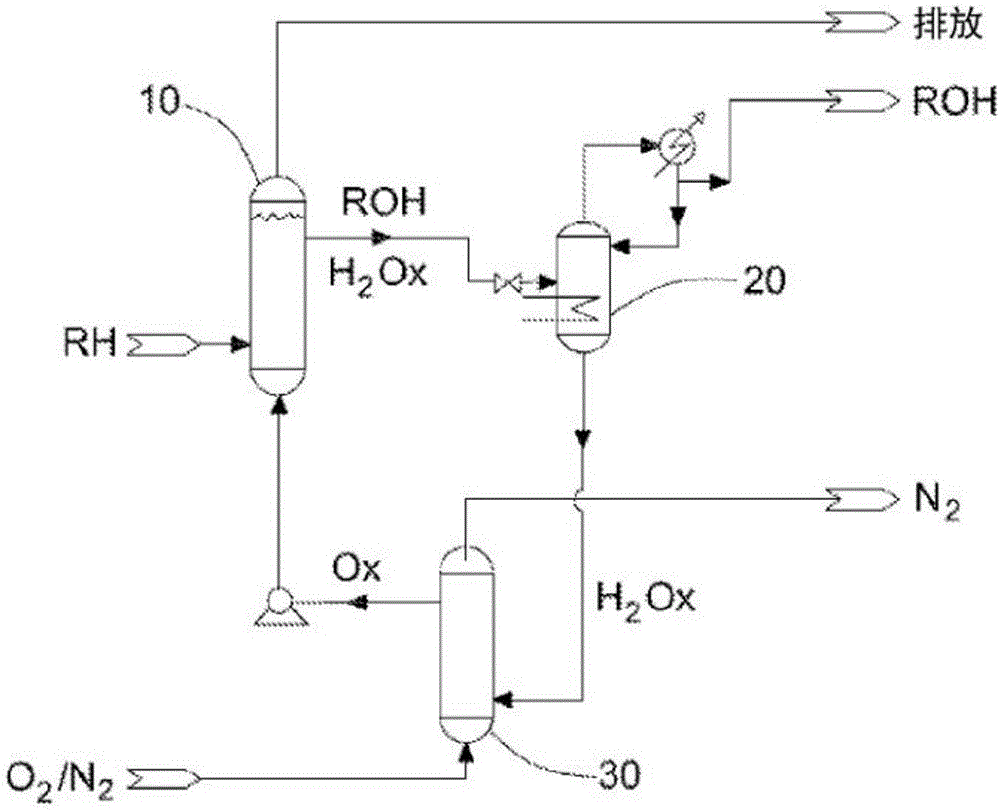
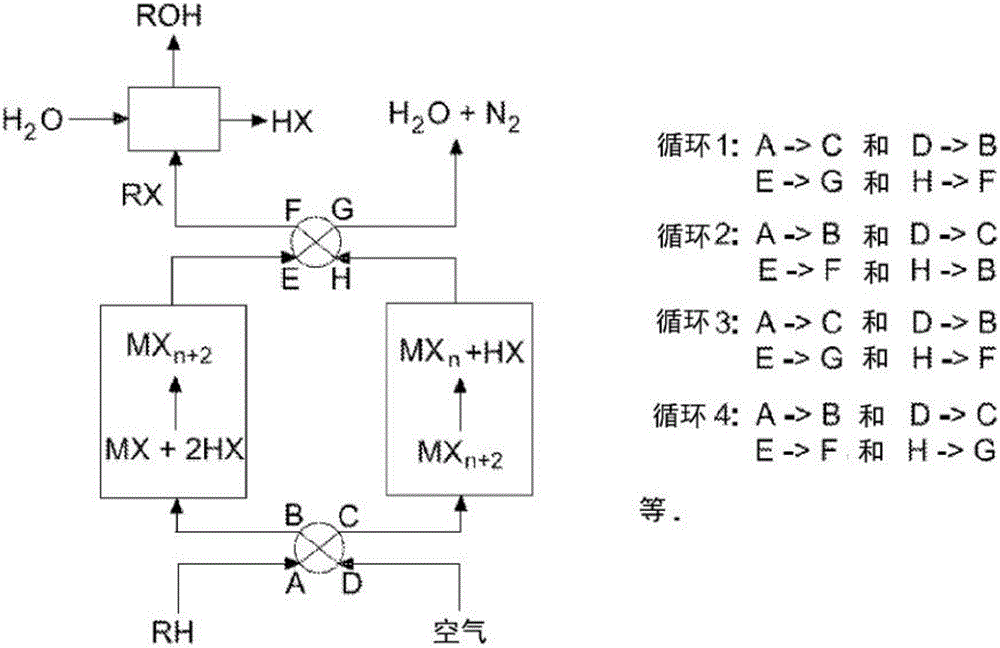
Process for the functionalization of heteroalkanes and arenes
A technology for functionalization and heteroalkane, which is applied in the field of functionalization of heteroalkane and aromatics, and can solve problems such as low selectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0167] This example illustrates the use of an oxidative electrophile comprising an oxidized form of a main group element to activate and functionalize the C-H bond of an arenebenzene.
[0168]found C. 6 f 5 I (+3) (TFA) 2 (1) Effectively used for selective monofunctionalization of benzene (PhH) to PhTFA at lower temperature (125 °C). Lowering the temperature of the reaction to 100 °C resulted in complete consumption of 1 and production of new species in solution, which was passed through 1 H-and 19 Preliminary identification by F-NMR of diaryl-λ 3 -Iodine [C 6 f 5 -I III -C 6 h 5 ][TFA](3), and only trace amounts of PhTFA and C 6 f 5 -I I (2). Further heating of the solution at 125 °C resulted in complete conversion of 3 to PhTFA and 2. This observation is consistent with the role of 3 as an intermediate in the conversion of PhH to PhTFA (Scheme 7).
[0169] Option 7
[0170]
Embodiment 2
[0172] This example illustrates the use of an oxidizing electrophile comprising an oxidized form of a main group element to activate and functionalize the C-H bond of the arene toluene.
[0173] Toluene and C 6 f 5 I (+3) (TFA) 2 (1) Reactivity. Using standard reaction conditions (eg 3h, 150°C), p-MeC was observed 6 h 4 TFA and o-MeC 6 h 4 TFA was produced quantitatively in an approximately 3:1 ratio (based on starting [1]); and did not form any benzylic oxygen functionalized product as expected from a free radical route (Scheme 8).
[0174] Option 8
[0175]
[0176] Clearly, relative to the methyl sp 3 - Functionalization of hybridized carbon atoms, sp of aryl rings 2 - Functionalization of hybridized carbon atoms is advantageous.
Embodiment 3
[0178] This example illustrates the use of an oxidative electrophile comprising an oxidized form of a main group element to activate and functionalize the C-H bond of an arenebenzene.
[0179] Make Pb(TFA) at 25°C 4 Reaction with benzene (PhH) in trifluoroacetic acid (TFAH) afforded the selectively monofunctionalized product PhTFA in 80% yield (Scheme 9). The reaction is very easy as the reaction occurs almost instantly at room temperature.
[0180] Option 9
[0181]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com