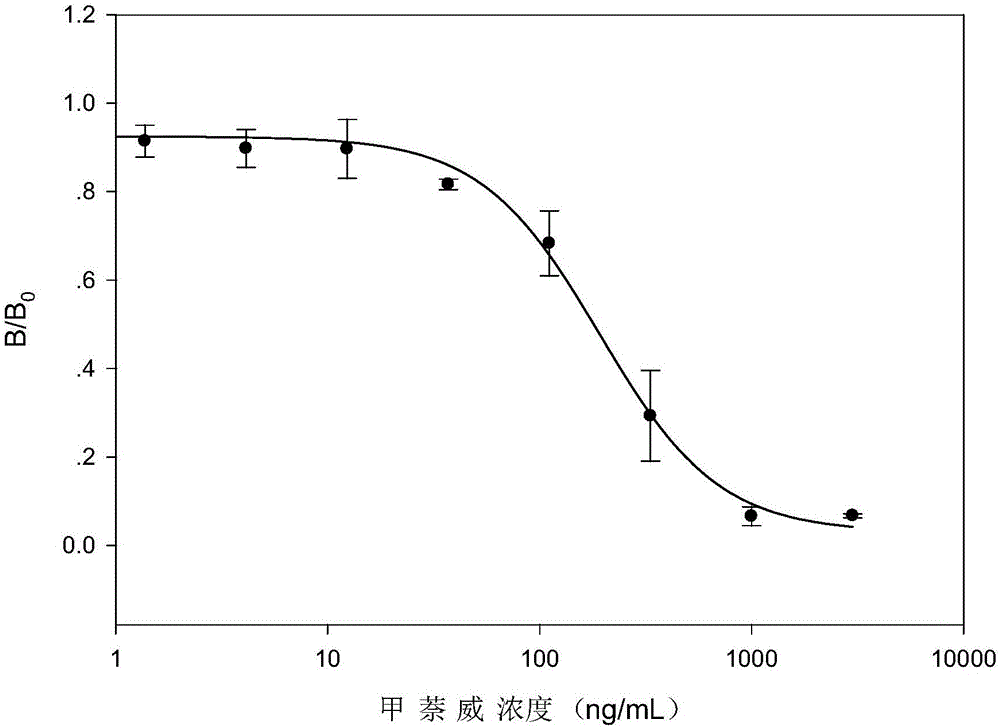
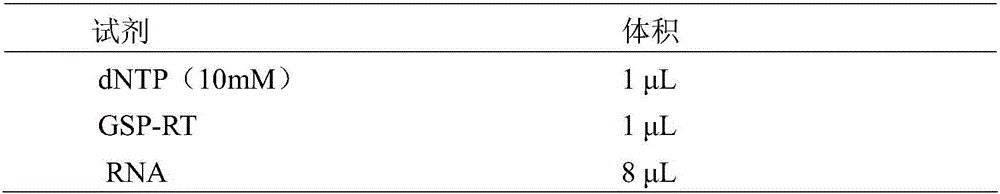
ELISA (enzyme-linked immuno sorbent assay) detection kit for analyzing residual carbaryl and application thereof
A carnacarb and kit technology, which is applied in the field of ELISA detection kits for analyzing carnacarb residues, can solve the problems of complex pretreatment, low sensitivity and high cost of the analysis method, and achieves a simple pretreatment process and less time-consuming. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] Example 1 Synthesis of the hapten N-(1-naphthyloxycarbonyl)-6-aminocaproic acid
[0022] 5.6g of NaOH (0.139mol) was dissolved in 56mL of distilled water, and 20g of α-naphthol (0.139mol) was added. The mixture was stirred at 85 °C for 1 h and cooled to room temperature. An excess of triphosgene (19.3 g of triphosgene and 100 mL of toluene) was slowly added in a fume hood, and the reaction was stirred at room temperature for 1 h. Anhydrous Na for organic phase 2 SO 4 After drying, the organic solvent was removed under reduced pressure to give a brown oil. It was dissolved in dichloromethane, vacuum distilled (1 mm Hg), and the fraction at 100°C was collected to obtain 13.7 g of the product naphthyl chloroformate as a pale yellow oil.
[0023] Dissolve 7.37g of 6-aminocaproic acid (56.1mmol) in 7.5mL of 4mol / L NaOH and cool to 4°C. Dissolve 6.25g of naphthyl chloroformate (30.3mmol) in 11mL of 1,4-dioxane, cool to 4°C, then mix with 7.5mL of 4mol / L cold NaOH, divide...
Embodiment 2
[0024] Example 2 Preparation of Carbaryl Coated Antigen
[0025] A conjugated complex was prepared with the hapten N-(1-naphthyloxycarbonyl)-6-aminocaproic acid and bovine serum albumin as the coating antigen. The preparation method is as follows:
[0026] (1) Dissolve 30.2mg of N-(1-naphthyloxycarbonyl)-6-aminocaproic acid, 13.9mg of N-hydroxysuccinimide and 24.3mg of N,N'-dicyclohexylcarbodiimide in 1mL of anhydrous In dimethylformamide, the reaction was stirred overnight at room temperature. The reaction solution was centrifuged (5000 rpm, 10 min), the precipitate was discarded, and the supernatant was collected, which contained active ester. (2) Dissolve 20mg of bovine serum albumin in 2mL of 0.05M, pH 9.6 carbonate buffer solution, add dropwise to the supernatant under stirring, and slowly add in about 20min. Then the stirring reaction was continued at room temperature for 4 h. (3) After the reaction, the reaction solution was put into a dialysis bag and dialyzed with...
Embodiment 3
[0027] Example 3 Construction of Carbaryl Phage Display Nanobody Library
[0028] The active ester method is used to couple the hapten of Example 1 to keyhole limpet hemocyanin, and the specific method is as follows:
[0029]Dissolve equimolar amounts of N-(1-naphthyloxycarbonyl)-6-aminocaproic acid, NHS and DCC in DMF, and stir overnight at room temperature for reaction. The reaction solution was centrifuged to discard the precipitate, and the supernatant was the active ester. The supernatant was added into the keyhole limpet hemocyanin solution under stirring, and the stirring reaction was continued for 4 h at room temperature. The reaction solution was put into a dialysis bag and dialyzed with PBS. Centrifuge, collect the supernatant, and freeze-dry to obtain the conjugate of N-(1-naphthyloxycarbonyl)-6-aminocaproic acid and keyhole limpet hemocyanin.
[0030] Dissolve 1mg of the conjugate in 1mL of normal saline, mix with 1mL of complete Freund's adjuvant, fully emulsif...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com