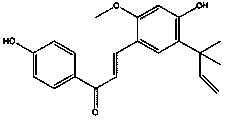
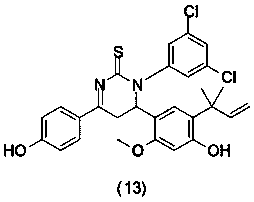
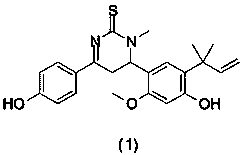
Licochalcone A thiouracil derivatives with antitumor activity and synthesis method thereof
The technology of a licorice chalcone and a synthetic method, which is applied in the field of medicinal chemistry, can solve problems such as poor water solubility, and achieve the effects of low production cost, mild reaction conditions, and environmentally friendly raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0021] A kind of synthetic method of licochalcone A thiouracil derivatives comprises the following steps:
[0022] (1) Add licochalcone A and thiourea compounds into the reactor at a molar ratio of 1:1~1:1.5, add toluene or DMF and mix evenly, and the solvent volume is less than 2 / 3 of the reactor volume, Add organic base catalyst triethylamine, stir on a magnetic stirrer, heat to 80°C~120°C, and reflux for 3~8 hours;
[0023] (2) During the reaction process, use thin-layer chromatography to track and monitor the progress of the reaction in time. After the raw materials are completely reacted, stop heating and remove the condensing device;
[0024] (3) Concentrate the solid-liquid mixture of the reaction system in step (2) under reduced pressure, separate and purify by column chromatography, and dry to obtain the target product.
[0025] The compound structural formula in some preferred embodiments of the present invention is as follows:
[0026]
[0027]
[0028]
Embodiment 1
[0030] 6-(4-Hydroxy-2-methoxy-5-(2-methylbut-3-en-2-yl)phenyl)-4-(4-hydroxyphenyl)-1-methyl-5 , Preparation of 6-dihydropyrimidine-2(1H)-thione (1).
[0031] In reactor, add 200mg (1mmol) licochalcone A and 68.44mg (1.3mmol) 1-methylthiourea, add 50ml toluene and 5mLDMF as reaction solvent, add 0.5mL triethylamine as catalyst, electric heating mantle is heated to 100°C, magnetically stirred and refluxed for 3 hours. The reaction was tracked by thin-layer chromatography. After the reaction was completed, it was concentrated under reduced pressure, and column chromatography was used to obtain a brown powder (113 mg). The total yield was 46.57%.
[0032]
[0033] Brown yellow crystalline powder solid. 1 H NMR(400MHz,DMSO-d6,300K):d 7.85(2H,d,J=7.52Hz), 6.95(1H,s), 6.85(2H,d,J=7.52Hz), 6.41(1H,s) , 6.30(1H,q), 5.35(2H,s), 5.00-4.98(2H,m), 3.9(1H,t), 3.83(3H,s), 3.04(3H,s), 1.76-1.51(2H ,m), 1.69(6H,s). 13 C NMR(75MHz,DMSO-d6)δ(ppm): 182.2, 164.6, 160.8, 156.1, 155.0, 148.8...
Embodiment 2
[0035] 1-Benzyl-6-(4-hydroxy-2-methoxy-5-(2-methylbut-3-en-2-yl)phenyl)-4-(4-hydroxyphenyl)-5 , Preparation of 6-dihydropyrimidine-2(1H)-thione (2).
[0036] In reactor, add 200mg (1mmol) licochalcone A and 127.73mg (1.3mmol) 1-benzylthiourea, add 50ml toluene and 5mLDMF as reaction solvent, add 0.5mL triethylamine as catalyst, electric heating mantle is heated to 100°C, magnetically stirred and refluxed for 5 hours. The reaction was tracked by thin-layer chromatography. After the reaction was completed, it was concentrated under reduced pressure, followed by column chromatography and dried to obtain a brown powder (124.72mg), with a total yield of 43.36%.
[0037]
[0038] Brown yellow crystalline powder solid. 1 H NMR(400MHz,DMSO-d6,300K):d 7.85(2H,d,J=7.52Hz), 7.33(2H,m),7.23(2H,m), 7.26(1H,m), 6.95(1H, s), 6.85(2H,d,J=7.52Hz),6.41(1H,s), 6.30(1H,q), 5.35(2H,s), 5.00-4.98(2H,m), 3.9(1H,t ), 3.83(3H,s),3.81(2H,s), 1.76-1.51(2H,m), 1.69 (6H,s). 13 C NMR(75MHz,DMSO-d6)...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com