Dual-function oxygen electrode catalyst containing non-noble-metal nanoparticles coated with nitrogen-doped porous carbon layer and preparation method of dual-function oxygen electrode catalyst
A bifunctional catalyst and nitrogen-doped porous carbon technology, which is applied in the field of non-noble metal catalysts and their preparation, can solve problems such as consumption, and achieve the effects of simple preparation process, increased effective surface area, and cheap raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
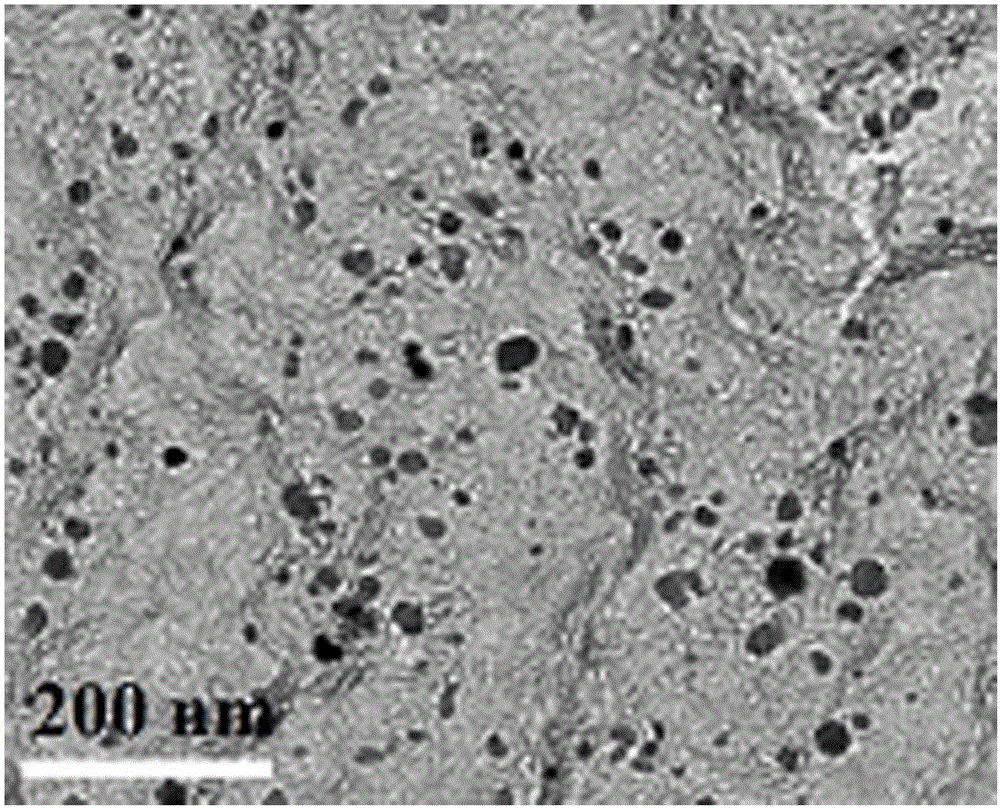
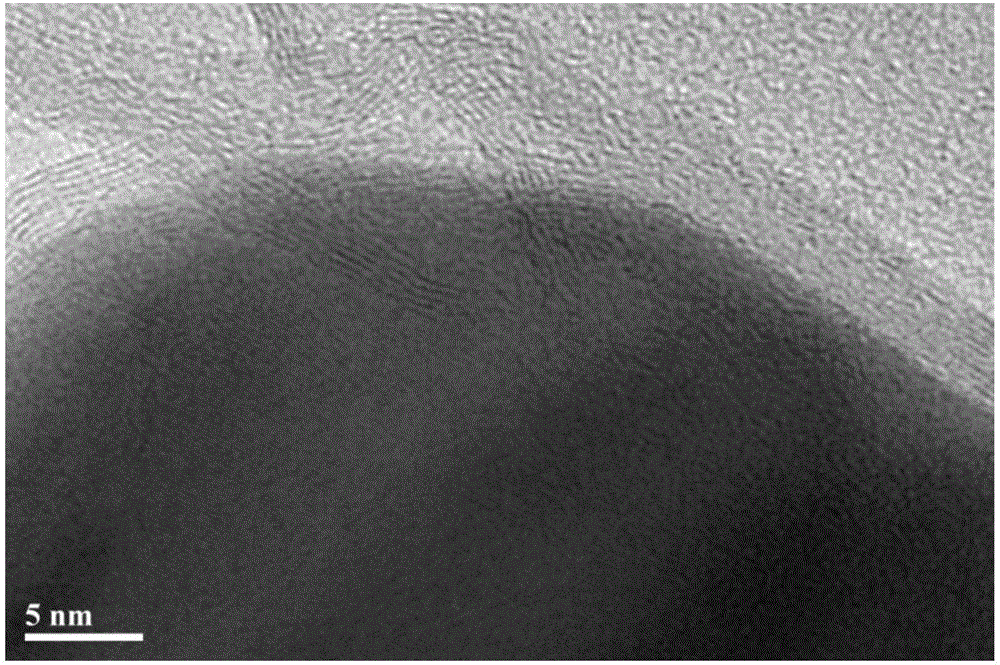
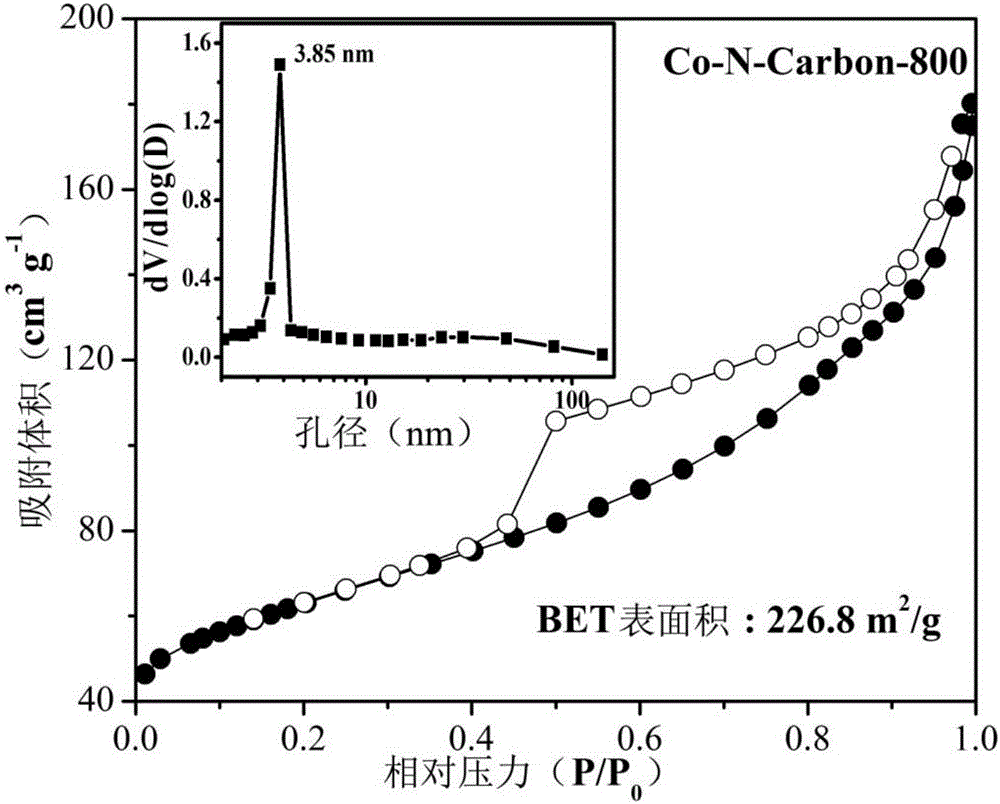
[0035] (1) Preparation of Co-N-Carbon-800 bifunctional oxygen electrode catalyst
[0036]Co-N-Carbon-800 preparation process is as follows: take 0.75g melamine, 0.5g (0.10g / mL) P123, 7.5mL (1wt%) Co(NO 3 ) 2 , 7.5mL of ultra-pure water, and the mixed solution was stirred and dispersed with a magnetic stirrer for 2 hours, and then ultrasonicated for 5 hours until the dispersion was uniform, and fully dried in a 60°C oven to obtain the precursor. The above precursor was ground into powder and placed in a quartz boat, under N 2 Under the protection of the atmosphere, the temperature was raised to 800°C at a heating rate of 5°C / min and kept for 2 hours, and then cooled to room temperature with the furnace to collect the product. Use 0.5mol / L H for the preliminary product 2 SO 4 Wash in the solution for 8 hours to remove unstable impurities, then wash the sample with deionized water until neutral, dry it in an oven at 60°C, and then grind it into powder to obtain Co-N-Carbon-80...
Embodiment 2
[0043] Preparation of Co-N-Carbon-700 Bifunctional Oxygen Electrode Catalyst
[0044] Co-N-Carbon-700 preparation process is as follows: take 0.80g dicyandiamide, 0.5g (0.10g / mL) P123, 8.0mL (1wt%) of CoSO 4 , 8.0mL of ultrapure water, and the mixed solution was first stirred and dispersed with a magnetic stirrer for 3 hours, and then ultrasonicated for 2 hours until the dispersion was uniform, and fully dried in a 60°C oven to obtain the precursor. The above precursor was ground into powder and placed in a quartz boat, under N 2 Under the protection of the atmosphere, the temperature was raised to 700°C at a heating rate of 3°C / min for 2.5 hours, and then cooled to room temperature with the furnace to collect the product. Use 0.5mol / L H for the preliminary product 2 SO 4 Wash in the solution for 6 hours to remove unstable impurities, then wash the sample with deionized water until neutral, dry it in an oven at 60°C, and then grind it into powder to obtain Co-N-Carbon-700 b...
Embodiment 3
[0046] Preparation of Co-N-Carbon-900 Bifunctional Oxygen Electrode Catalyst
[0047] Co-N-Carbon-900 preparation process is as follows: Take 0.80g cyanamide, 0.5g (0.10g / mL) F127, 7.5mL (1wt%) of CoSO 4 , 8.0mL of ultrapure water, and the mixed solution was first stirred and dispersed with a magnetic stirrer for 3 hours, and then ultrasonicated for 3 hours until the dispersion was uniform, and fully dried in an oven at 80°C to obtain the precursor. The above precursor was ground into powder and placed in a quartz boat, under N 2 Under the protection of the atmosphere, the temperature was raised to 900°C at a heating rate of 5°C / min and kept for 1.5h, and then cooled to room temperature with the furnace to collect the product. Use 0.5mol / L H for the preliminary product 2 SO 4 Wash in the solution for 6 hours to remove unstable impurities, then wash the sample with deionized water until neutral, dry it in an oven at 80°C, and then grind it into powder to obtain Co-N-Carbon-9...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
| Thickness | aaaaa | aaaaa |
| Dimensions | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com