Medicinal composition for preventing and treating neonatal jaundice and preparation method and application thereof
A technology for neonatal jaundice and composition, applied in the field of pharmaceutical composition for preventing and treating neonatal jaundice and its preparation, and the field of pharmaceutical composition for preventing and treating neonatal jaundice, which can solve potential safety hazards, and there is no newborn drug safety data and clinical experiment data and other problems, to achieve the effect of accelerated self-healing, clear pharmacology, and definite curative effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] Example 1 Preparation of freeze-dried oral powder
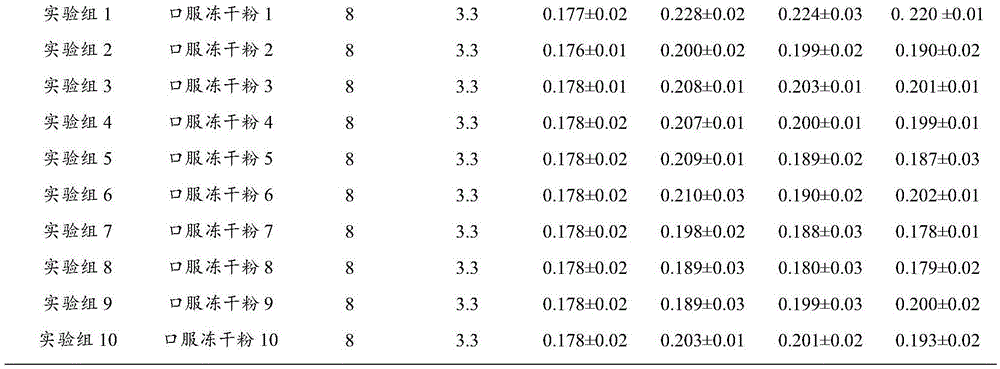
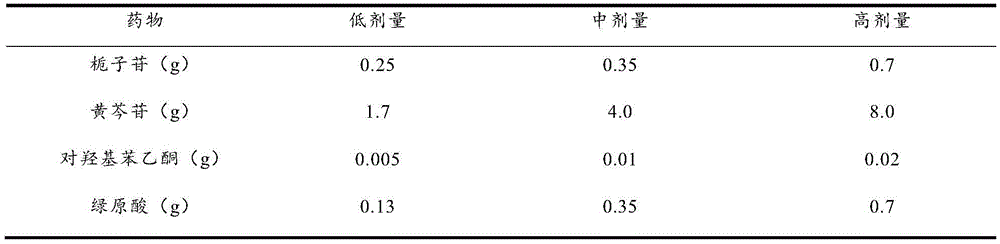
[0035] Weigh 0.25g of geniposide, 4.0g of baicalin, 0.01g of p-hydroxyacetophenone, and 0.35g of chlorogenic acid, dissolve them in 800ml of purified water, adjust the pH value to 7.0 with 10% NaOH, add purified water to 1000ml, and filter , Packed into vials, 1ml per bottle. Move the vials to a vacuum freeze dryer for freeze drying. The temperature drops to -40°C within 1 hour, after 2 hours of heat preservation, the vacuum pump is turned on, the vacuum degree is maintained at 300 Pa, and the temperature is gradually raised to -5°C within 10 hours, the vacuum degree is reduced to 10 Pa, and the temperature is raised to +35°C after maintaining for 2 hours. After maintaining for 3 hours, the vacuum degree does not change, and then it is out of the box, capped, inspected, and packaged. Product moisture is not more than 1%.
Embodiment 2
[0036] Example 2 Preparation of freeze-dried oral powder
[0037] Weigh 0.25g of pregeniposide, 1.7g of baicalin, 0.005g of p-hydroxyacetophenone, and 0.13g of chlorogenic acid, dissolve them in 800ml of purified water, adjust the pH value to 7.0 with 10% NaOH, and then add purified water to 1000ml. Filter and distribute to vials, 1ml per bottle. Move the vials to a vacuum freeze dryer for freeze drying. The temperature drops to -40°C within 1 hour, after 2 hours of heat preservation, the vacuum pump is turned on, the vacuum degree is maintained at 300 Pa, and the temperature is gradually raised to -5°C within 10 hours, the vacuum degree is reduced to 10 Pa, and the temperature is raised to +35°C after maintaining for 2 hours. After maintaining for 3 hours, the vacuum degree does not change, and then it is out of the box, capped, inspected, and packaged. Product moisture is not more than 1%.
Embodiment 3
[0038] Example 3 Preparation of freeze-dried oral powder
[0039] Weigh 0.7g of geniposide, 8.0g of baicalin, 0.02g of p-hydroxyacetophenone, and 0.7g of chlorogenic acid, dissolve them in 800ml of purified water, adjust the pH value to 7.0 with 10% NaOH, add purified water to 1000ml, and filter , Packed into vials, 1ml per bottle. Move the vials to a vacuum freeze dryer for freeze drying. The temperature drops to -40°C within 1 hour, after 2 hours of heat preservation, the vacuum pump is turned on, the vacuum degree is maintained at 300 Pa, and the temperature is gradually raised to -5°C within 10 hours, the vacuum degree is reduced to 10 Pa, and the temperature is raised to +35°C after maintaining for 2 hours. After maintaining for 3 hours, the vacuum degree does not change, and then it is out of the box, capped, inspected, and packaged. Product moisture is not more than 1%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com