Method for preparing 6-bromotriphenylphosphonio-n-caproic acid
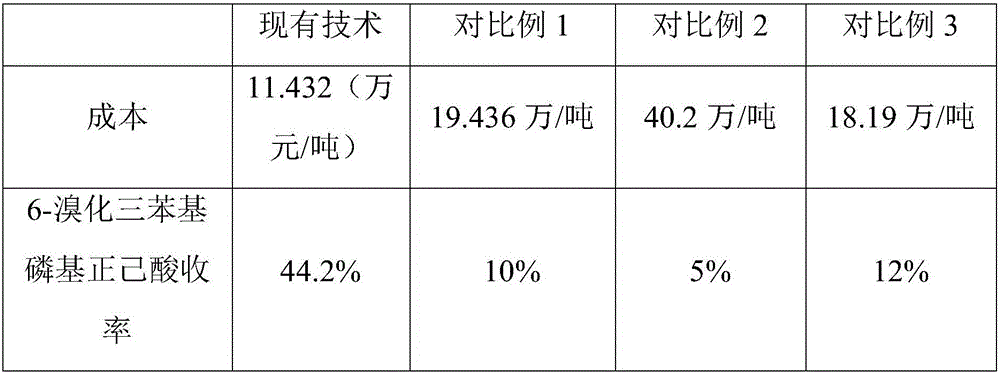
A technology of triphenylphosphine bromide and n-hexanoic acid, which is applied in the fields of chemical instruments and methods, organic chemistry, and compounds of Group 5/15 elements of the periodic table, can solve the problems of pollution and high cost, and achieve cost reduction, Effect of increasing yield and reducing production cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
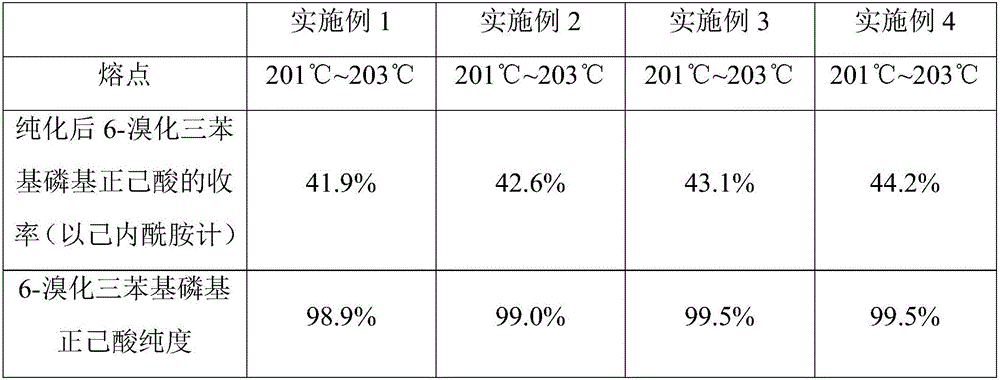
Embodiment 1
[0049] The present embodiment provides a kind of preparation method of 6-bromotriphenylphosphoryl n-hexanoic acid, the method comprises the following steps:
[0050] 1. Hydrolysis reaction: put 220g of caprolactam and 140g of sodium hydroxide into a 500ml bottle, heat up and reflux for 5 hours, after the reaction is complete, add 230g of water to dilute to obtain a hydrolyzate.
[0051] Add 353g of hydrolyzate to the 1000ml bottle, add dropwise 230g of 30% industrial hydrochloric acid to adjust the pH to 4, pour it out for later use.
[0052] 2. Diazotization reaction: Add 150g of water and 100g of sodium nitrite into a 1000ml bottle, stir to dissolve, lower to -5°C, add the hydrolyzate with adjusted pH dropwise at -5°C to 0°C, and finish adding dropwise in 35 minutes. After the dropwise addition, keep it warm at -5°C to 0°C for 5 hours, then add 42g of 30% industrial hydrochloric acid dropwise, heat up and reflux for 13min until the starch potassium iodide test paper does not...
Embodiment 2
[0057] The present embodiment provides a kind of preparation method of 6-bromotriphenylphosphoryl n-hexanoic acid, the method comprises the following steps:
[0058] 1. Hydrolysis reaction: Put 230g caprolactam and 150g sodium hydroxide into a 500ml bottle, heat up and reflux for 7 hours, after the reaction is complete, add 250g water to dilute to obtain a hydrolyzate.
[0059] Add 353g of hydrolyzate to the 1000ml bottle, add dropwise 245g of 30% industrial hydrochloric acid to adjust the pH to 5, pour it out for later use.
[0060] 2. Diazotization reaction: Add 157g of water and 107g of sodium nitrite into a 1000ml bottle, stir to dissolve, lower to -8°C, add the hydrolyzate with adjusted pH dropwise at -8°C to 0°C, and finish adding dropwise in 40 minutes. After the dropwise addition, keep it warm at -8°C to 0°C for 6 hours, then add 50g of 30% industrial hydrochloric acid dropwise, heat up and reflux for 19min until the starch potassium iodide test paper does not turn blue,...
Embodiment 3
[0065] The present embodiment provides a kind of preparation method of 6-bromotriphenylphosphoryl n-hexanoic acid, the method comprises the following steps:
[0066] 1. Hydrolysis reaction: put 223g caprolactam and 135g sodium hydroxide into a 500ml bottle, heat up and reflux for 7 hours, after the reaction is complete, add 240g water to dilute to obtain a hydrolyzate.
[0067] Add 350g of hydrolyzate to the 1000ml bottle, add dropwise 238g of 30% industrial hydrochloric acid to adjust the pH to 4.7, pour it out for later use.
[0068] 2. Diazotization reaction: Add 154g of water and 104g of sodium nitrite into a 1000ml bottle, stir to dissolve, lower to -2°C, add the hydrolyzate with adjusted pH dropwise at -2°C to 0°C, and finish adding dropwise in 40 minutes. After the dropwise addition, keep it warm at -2°C to 0°C for 7 hours, then add 52g of 30% industrial hydrochloric acid dropwise, heat up and reflux for 13 minutes until the starch potassium iodide test paper does not t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com