Process for preparing indobufen
A technology based on indobufen and isoindoline, which is applied in the field of preparation of indobufen, can solve the problems of being unsuitable for industrial production, high toxicity, and difficulty in dissolving residues, so as to be beneficial to the health protection of employees, Low impurity content, the effect of environmental protection workers' health protection
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] Embodiment 1, hydrogenation reaction and cyclization reaction
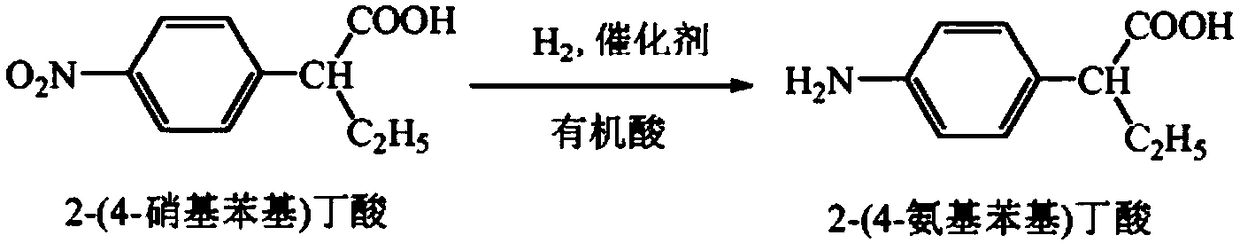
[0029] Add 85kg of 2-(4-nitrophenyl)butyric acid, 2.2kg of Pd / C catalyst, and 850L of acetic acid into the hydrogenation reactor, start stirring, and feed hydrogen to control the hydrogen pressure at 1.0Mpa and the reaction temperature at 25°C , carry out the hydrogenation reaction, keep the hydrogen pressure at 1.0Mpa, when the hydrogen is no longer consumed, stop the reaction, filter to obtain 2-(4-aminophenyl)butyric acid filtrate.
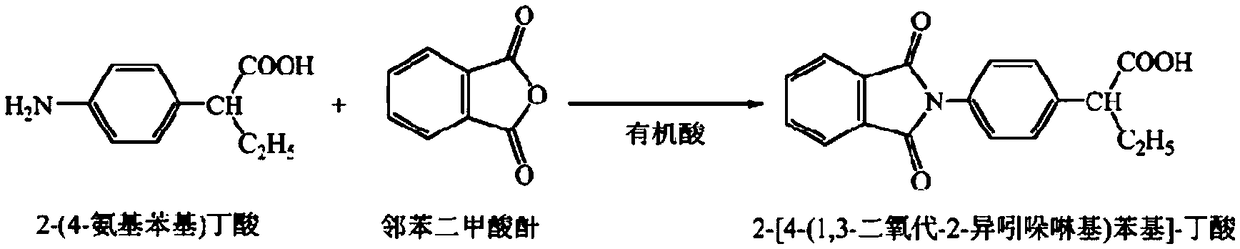
[0030] Put the 2-(4-aminophenyl)butyric acid filtrate into the reaction kettle, when the temperature rises to 40°C, slowly add 136 kg of phthalic anhydride, continue to heat up to 80°C, stir for 3 hours, and the cyclization reaction ends . Cool down to 25°C, centrifuge and dry to obtain 113.1 kg of 2-[4-(1,3-dioxo-2-isoindolinyl]phenylbutyric acid, yield 89.9%, mp 216~ 218°C.
Embodiment 2-5
[0031] Embodiment 2-5, hydrogenation reaction, cyclization reaction and comparative test
[0032] Following examples 2-5, all take 85 kilograms of 2-(4-nitrophenyl) butyric acid as starting reaction material. Except specifying, other materials, technique are identical with embodiment 1.
[0033]
[0034] In the above table, Example 5 wherein is the yield and purity after carrying out the industrial scale-up test according to the technique of prior art 2;
[0035] As can be seen from the above table, when the selection of materials and / or process conditions is outside the protection scope of the present application, the yield of intermediates is low, and the melting point is not within the standard, which indicates that more impurities may be produced .
Embodiment 6
[0036] Embodiment 6, zinc powder reduction reaction
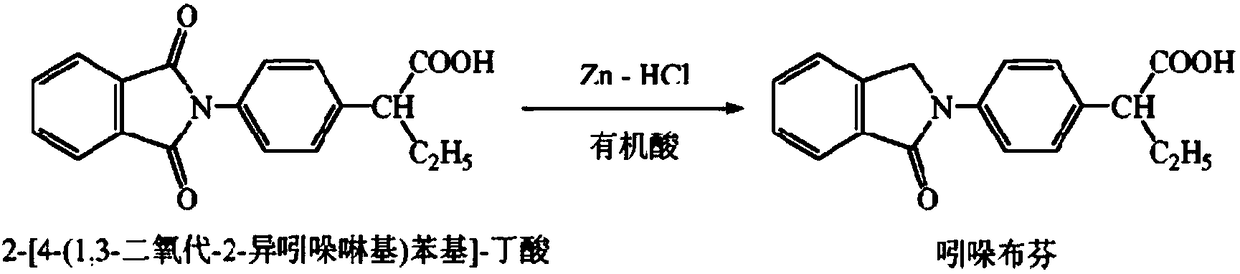
[0037] 80 kilograms of 2-[4-(1,3-dioxo-2-isoindolinyl] phenylbutyric acid and 480L acetic acid prepared by the method of Example 1 were dropped into a reaction tank, and the stirring was started, and 64 kilograms of Put the zinc powder into the reaction tank, feed hydrogen chloride gas under normal pressure, and control the reaction temperature at 82°C. The reaction starts, the reaction time is 1 hour, and the reaction feed liquid is all clarified. Filter, wash the filter residue with acetic acid, combine the filtrate and washing liquid, and decompress Recover the solvent, pour the residual liquid into an appropriate amount of water, stir, adjust the pH to 5.5-6.5 with ammonia water, and centrifuge and dry to obtain 72.3 kg of indobufen crude product, with a yield of 94.7%, a purity of 98.0%, a simple impurity content of less than 1.2%, and mp 182- 183°C.
[0038] After the crude product of indobufen is refined with activa...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com