A small molecule fluorescent probe for rapid recognition of superoxide, its preparation method and application
A fluorescent probe, superoxide root technology, applied in fluorescence/phosphorescence, chemical instruments and methods, luminescent materials, etc., can solve the problems of long detection time, inability to realize cell imaging, etc., achieve simple post-processing process, resistance to other molecules Strong interference ability and good selectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] Synthesis of probe compound NS-O:
[0022]
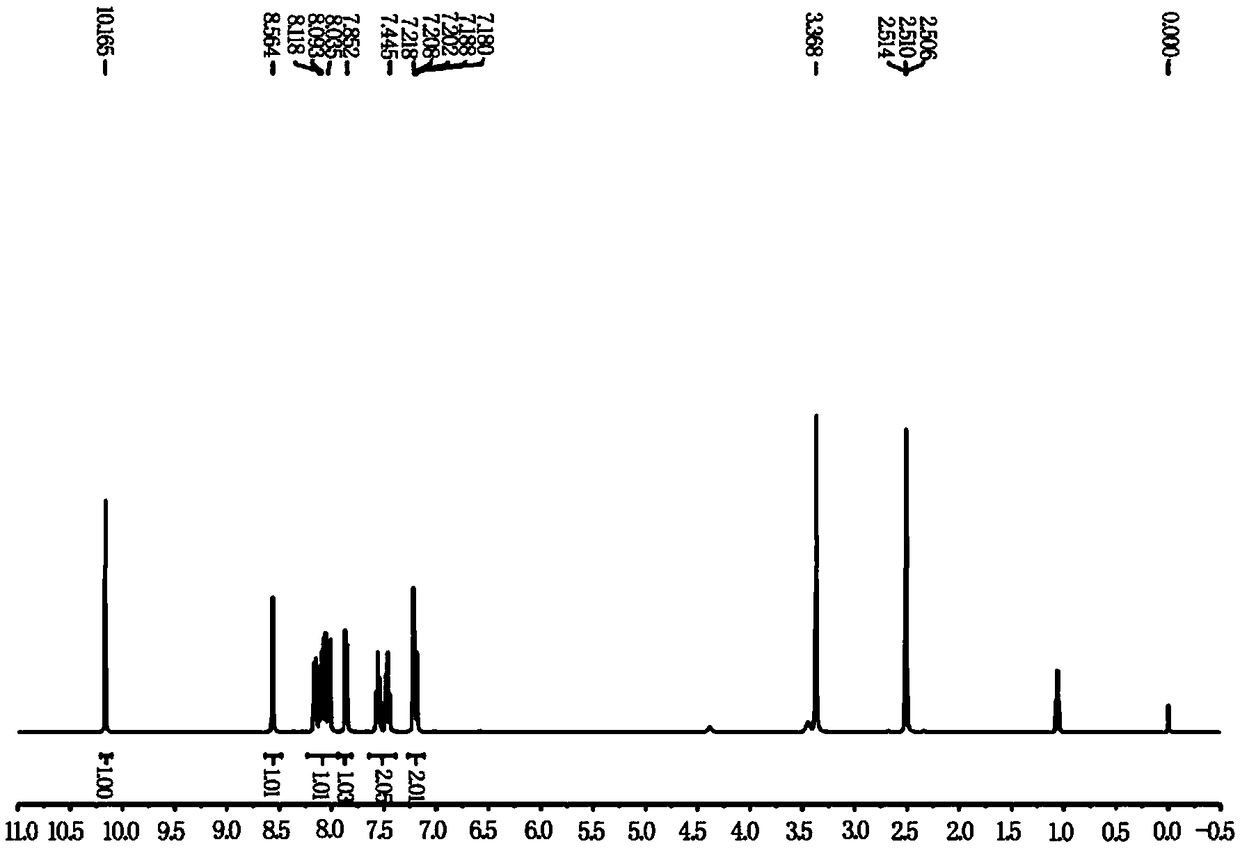
[0023] Compound 1 (0.5mmol, 86.0mg) and compound 2 (1.1equiv, 68.8mg) were added to the reaction flask under a nitrogen atmosphere, and then ethanol (5.0mL) was added to the above reactor at once, under nitrogen atmosphere After heating and refluxing for 5 hours, the reaction was detected by dot plate until the raw materials disappeared, the solvent was spin-dried under reduced pressure to obtain a crude product, and the probe compound NS-O was separated by silica gel column. The silica gel particle size was 200-300 mesh, and the yield was 63%. 1 H-NMR (400MHz, DMSO-d 6 )δ10.12(s,1H),8.56(s,1H),8.17-8.01(m,4H),7.86(d,J=8.8Hz,1H),7.58-7.45(m,2H),7.22-7.18 (m,2H). The probe's 1 H NMR chart see figure 1 .
Embodiment 2
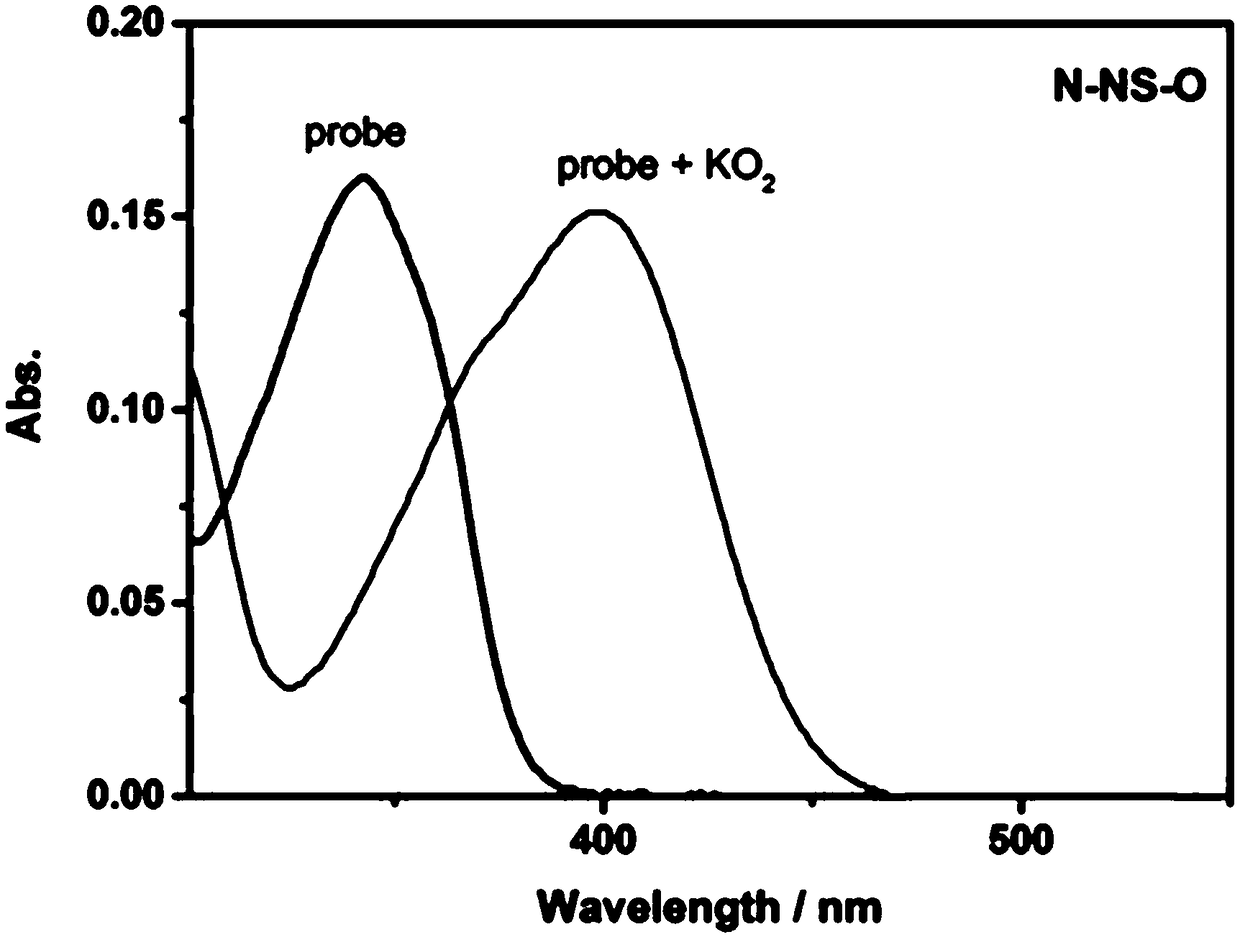
[0025] Probe NS-O with O 2 - Add its visible light absorption change
[0026] The probe NS-O prepared in Example 1 was dissolved in N,N-dimethylformamide (DMF) to prepare a 1 mmol / L stock solution. Take 30μL from the stock solution and add it to a 5mL centrifuge tube, measure its absorption spectrum, and then add O 2 - (10equiv) Standard solution, diluted to 3mL (10μM) with DMSO / PBS (1:1, v / v), and measure its absorption spectrum. See the spectrogram figure 2 Shown. by figure 2 It can be seen that after adding probe NS-O, directly add O 2 - , The absorption change can be detected instantaneously, and O 2 - Detection.
Embodiment 3
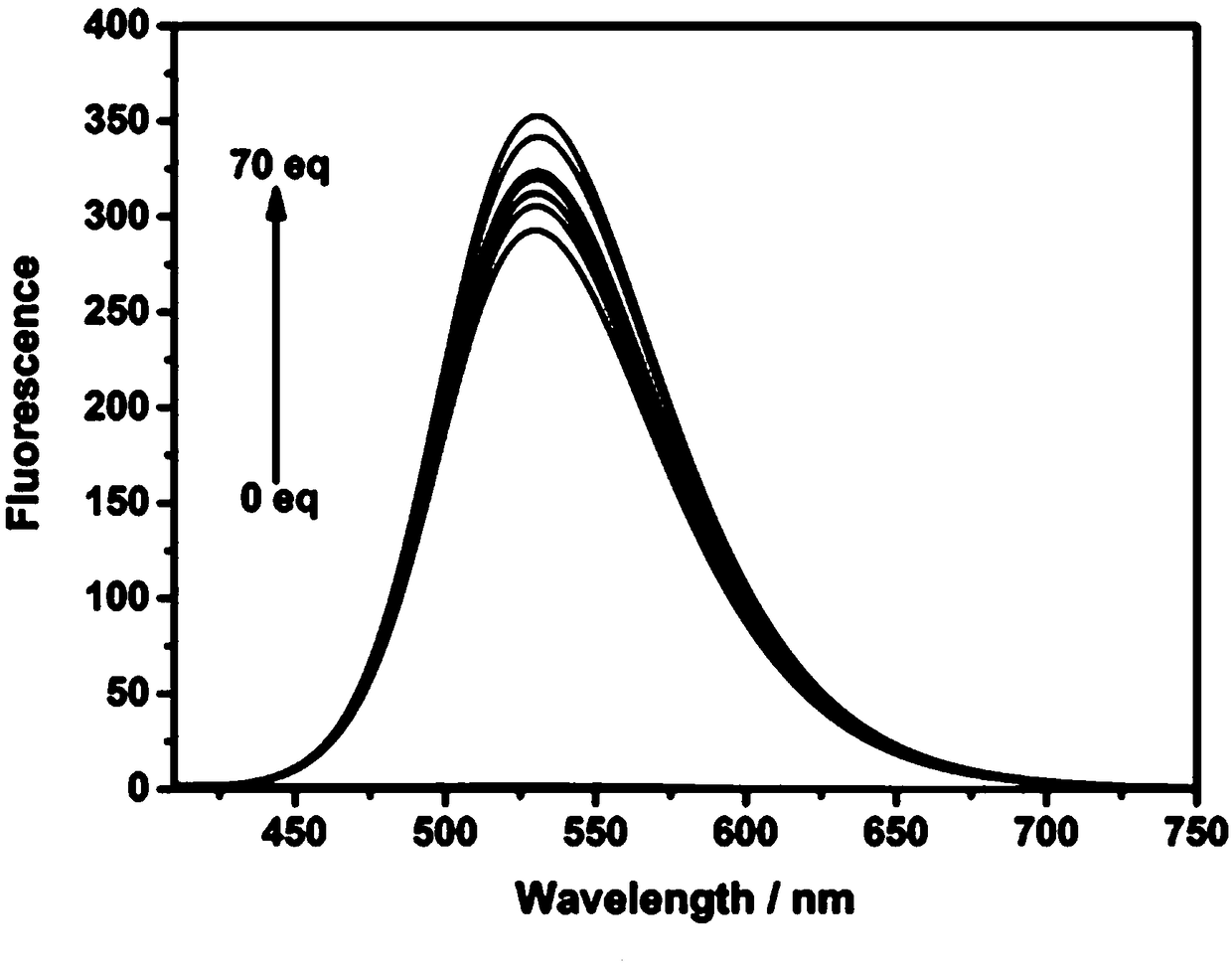
[0028] Probe compound NS-O fluorescent probe with O 2 - Change of fluorescence spectrum with increase in equivalent
[0029] Take 30μL from the stock solution of Example 2 and add it to a 5mL centrifuge tube, add different equivalents (0-70equiv) of O 2 - The standard solution was diluted to 3 mL with a 1:1 volume ratio of PBS buffer solution (0.1mol / L, pH=7.4) and DMSO, and 400nm was used as excitation light to measure its fluorescence properties. Fluorescence spectra such as image 3 Shown. by image 3 It can be seen that with O 2 - Fluorescence gradually increased with the increase of the added equivalent.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com