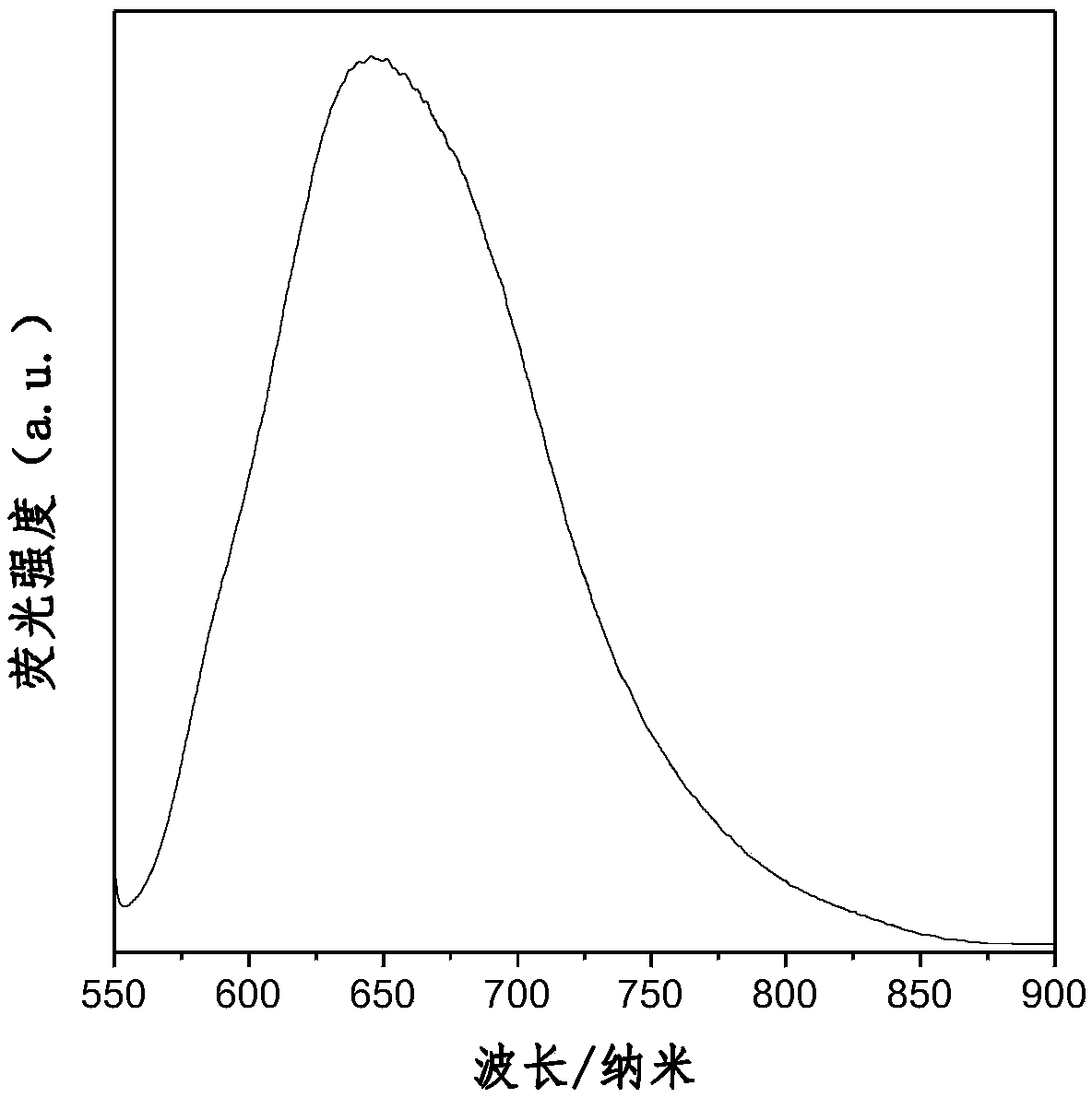
Aggregation-induced red light-emitting material and preparation method thereof
An aggregation-induced, red-emitting technology, applied in luminescent materials, chemical instruments and methods, organic chemistry, etc., can solve the problems of harsh reaction conditions, aggregation and quenching of organic red-light materials, and expensive, and achieve mild reaction conditions and experimental results. Step-by-step effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0053] In a preferred technical solution of the present invention, R 1 is n-butyl, R 2 for -H.
[0054] (1) Synthesis of intermediate 1-(4-hydroxyphenyl)-1,2,2-triphenylethylene. 4-Hydroxybenzophenone (1.9 g, 10 mmol), benzophenone (2.2 g, 12 mmol), and Zn powder (2.9 g, 44 mmol) were added to a 250 mL round bottom flask. After vacuuming and changing nitrogen three times, 80 mL of tetrahydrofuran was added. After cooling to 0°C, slowly add TiCl 4 (4.2 g, 22 mmol), followed by stirring for 1 h. Then stir at 70°C for 24h, after cooling to room temperature, add 80mL of dilute hydrochloric acid (1mol / L) to adjust to neutrality, extract three times with DCM, collect the organic layer, dry over anhydrous magnesium sulfate, and spin the solvent to obtain the crude product. A 20:1 mixture of petroleum ether and ethyl acetate as eluent, SiO 2 As a stationary phase, 0.8 g of a white solid was obtained by column chromatography, and the yield was 47%.
[0055]
[0056] (2) Synth...
Embodiment 2
[0064] In another preferred technical solution of the present invention, R 1 is cyclohexyl, R 2 for -CH 3 .
[0065] (1) Synthesis of intermediate 4-(1,2-diphenyl-2-p-tolylvinyl)phenol. 4-Hydroxybenzophenone (1.9 g, 10 mmol), 4-methylbenzophenone (2.9 g, 15 mmol), and Zn powder (3.9 g, 60 mmol) were added to a 250 mL round bottom flask. After vacuuming and changing nitrogen three times, 80 mL of tetrahydrofuran was added. After cooling to 0°C, slowly add TiCl 4 (5.7g, 30mmol), followed by stirring for 0.8h. Then stir at 65°C for 36h, after cooling to room temperature, add 80mL of dilute hydrochloric acid (1mol / L) to adjust to neutrality, extract three times with DCM, collect the organic layer, dry over anhydrous magnesium sulfate, and spin the solvent to obtain the crude product. A 40:1 mixture of petroleum ether and ethyl acetate as eluent, SiO 2 As a stationary phase, 0.76 g of a white solid was separated by column chromatography, and the yield was 42%.
[0066]
...
Embodiment 3
[0073] In another preferred technical solution of the present invention, R 1 is 2-ethylhexyl, R 2 for-OCH 3 .
[0074] (1) Synthesis of intermediate 4-(2-(4-methoxyphenyl)-1,2-diphenylvinyl)phenol. Into a 250 mL round bottom flask were added 4-hydroxybenzophenone (1.9 g, 10 mmol), 4-methoxybenzophenone (2.1 g, 10 mmol), Zn powder (2.6 g, 40 mmol). After vacuuming and changing nitrogen three times, 80 mL of tetrahydrofuran was added. After cooling to 0°C, slowly add TiCl 4 (3.8g, 20mmol), then stirred for 1h, then stirred at 85°C for 12h, after cooling to room temperature, added 80mL of dilute hydrochloric acid (1mol / L) to adjust to neutral, extracted three times with DCM, collected the organic layer, anhydrous magnesium sulfate Drying, spin-drying solvent obtains crude product, is the eluent with the mixture of sherwood oil and ethyl acetate of 60:1 with the volume ratio, SiO 2 As a stationary phase, 0.87 g of a white solid was obtained by column chromatography, with a y...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com