Synthetic method of quinazolinone Schiff base compounds
A quinazolinone and a synthesis method technology are applied in the field of synthesizing quinazolinone Schiff base compounds, and can solve the problems of adding ligands, many reaction steps, high solvent toxicity, etc., so as to improve the reaction yield and simplify the The effect of simple and easy reaction steps and preparation methods
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0053] A kind of preparation method of quinazolinone Schiff base compound, synthetic route is as follows:
[0054]
[0055] R is H or Cl, R 1 and R 2 Selected from independent H, C1-C6 alkyl, benzyl, aromatic groups with different substituents, aromatic groups with different substituents include unsubstituted aryl, unsubstituted heteroaryl, aromatic with substituents A group or a heteroaryl group with substituents, the substituents are independently selected from C1-C6 alkyl, C1-C6 alkoxy.
[0056] Include the following steps:
[0057] Step 1: Weigh isatoic anhydride (as shown in formula I), carbonyl-containing compound (aromatic aldehyde, heterocyclic aromatic aldehyde or cyclic compound containing carbonyl in the molar ratio of 1:(2-2.2):1, such as formula III) and hydrazine hydrate (as shown in formula II) as raw materials;
[0058]Step 2: After mixing the raw materials weighed in step 1 in a solvent (ethanol, DMSO, DMF, Dioxane, NMP, water, or a mixed solvent obtain...
Embodiment 1
[0062] Preparation of 2-phenyl-3-(benzylideneamino)-4(1H)-quinazolinone
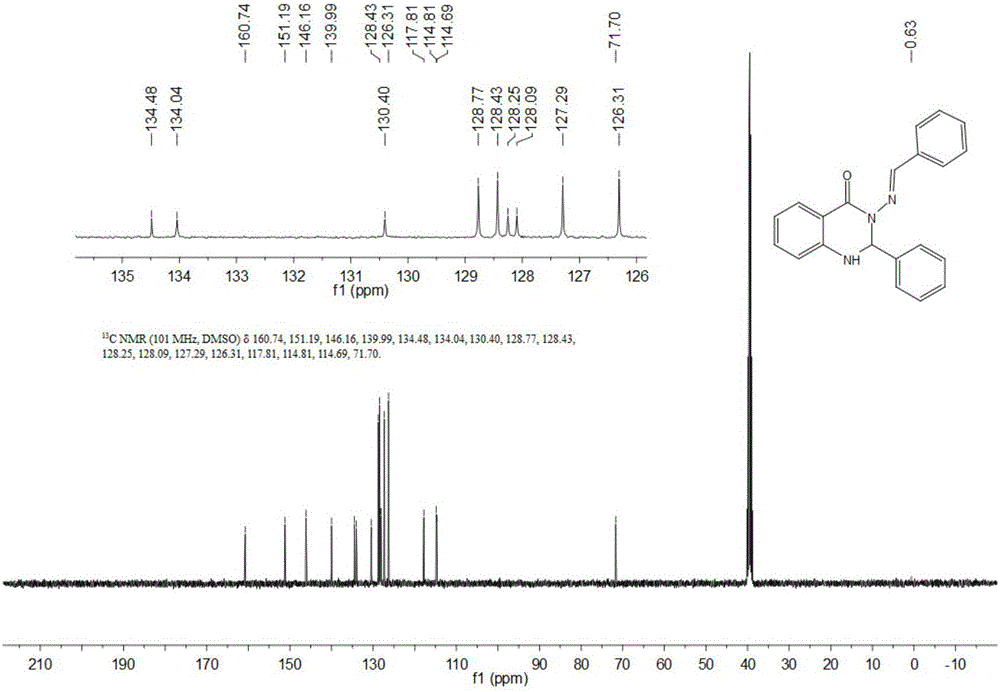
[0063] Dissolve 1 mmol of isatoic anhydride in ethanol and water mixed solution solvent with a volume ratio of 1:3, add 2.2 mmol of benzaldehyde and 1 mmol of hydrazine hydrate, use 0.15 mmol of nano-copper oxide as a catalyst, and heat at reflux at 76 ° C for 5 Hour, monitor the progress of reaction with TLC, concentrate after the reaction is complete, separate through column chromatography, obtain 271mg light yellow solid, productive rate is 83%, and gained product structural formula is as follows:
[0064]
[0065] Such as figure 1 with figure 2 Shown, product NMR characterization: 13 C NMR(101MHz,DMSO)δ160.74(s,1H),151.19(s,2H),146.16(s,1H),139.99(s,1H),134.48(s,1H),134.04(s,2H) ,130.40(s,2H),128.77(s,4H),128.53–127.95(m,9H),127.29(s,5H),126.31(s,5H),117.81(s,2H),114.75(d,J =11.4Hz,4H),71.70(s,2H). 13 C NMR (101MHz, DMSO) δ160.74, 151.19, 146.16, 139.99, 134.48, 134.04, 130.40, 128.77, 128.4...
Embodiment 2
[0067] Preparation of 2-phenyl-3-(4'-formyl-benzylideneamino)-4(1H)-quinazolinone
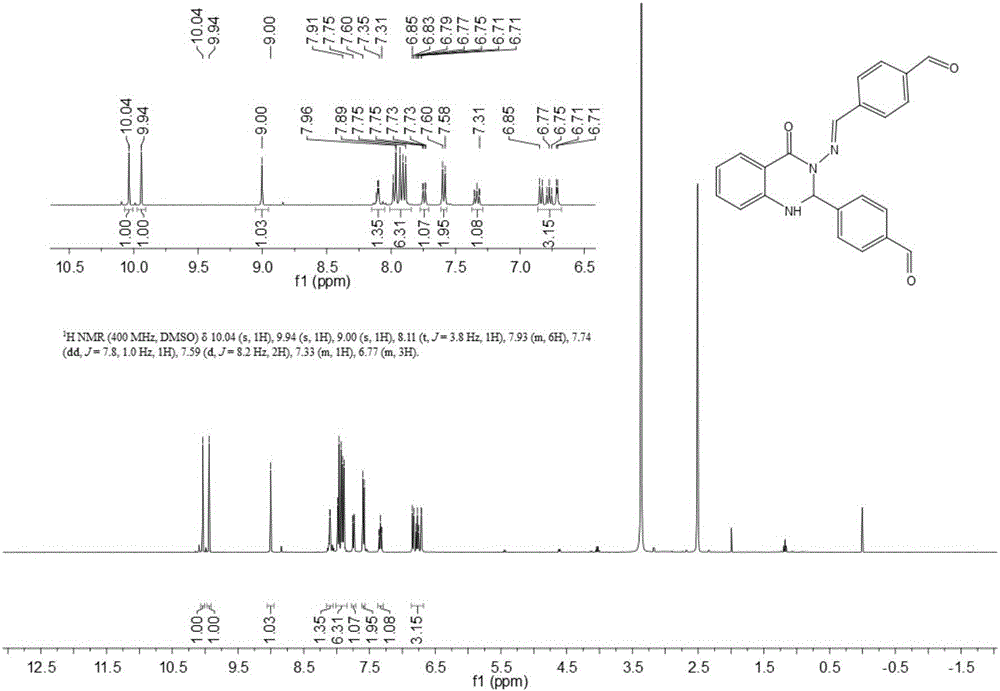
[0068] Dissolve 1mmol of isatoic anhydride in ethanol and water mixture solvent with a volume ratio of 1:2.5, add 2mmol of terephthalaldehyde and 1mmol of hydrazine hydrate, use 0.1mmol of nano-cerium oxide as a catalyst, and heat to reflux at 80°C After 6 hours, the reaction was monitored by TLC. After the reaction was complete, it was concentrated and separated by column chromatography to obtain 268 mg of a light yellow solid with a yield of 70%. The structural formula of the product obtained was as follows:
[0069]
[0070] Such as image 3 with Figure 4 Shown, product NMR characterization: 1 H NMR(400MHz,DMSO)δ10.04(s,1H),9.94(s,1H),9.00(s,1H),8.11(t,J=3.8Hz,1H),8.01–7.84(m,6H) ,7.74(dd,J=7.8,1.0Hz,1H),7.59(d,J=8.2Hz,2H),7.37–7.29(m,1H),6.86–6.68(m,3H). 13 C NMR (101MHz, DMSO) δ192.73, 192.54, 160.68, 148.95, 146.13, 145.95, 139.94, 137.00, 136.06, 134.44, 129.91, 129.75, 128.25, 1...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com