Minicircle DNA vector preparations and methods of making and using the same
A preparation and carrier technology, which is applied in the field of reducing the production cost of small rings, and can solve the problems of immunogenic virus complications, integration-mediated mutations, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0139] Reduction of impurity DNA in minicircle preparations from BW27783
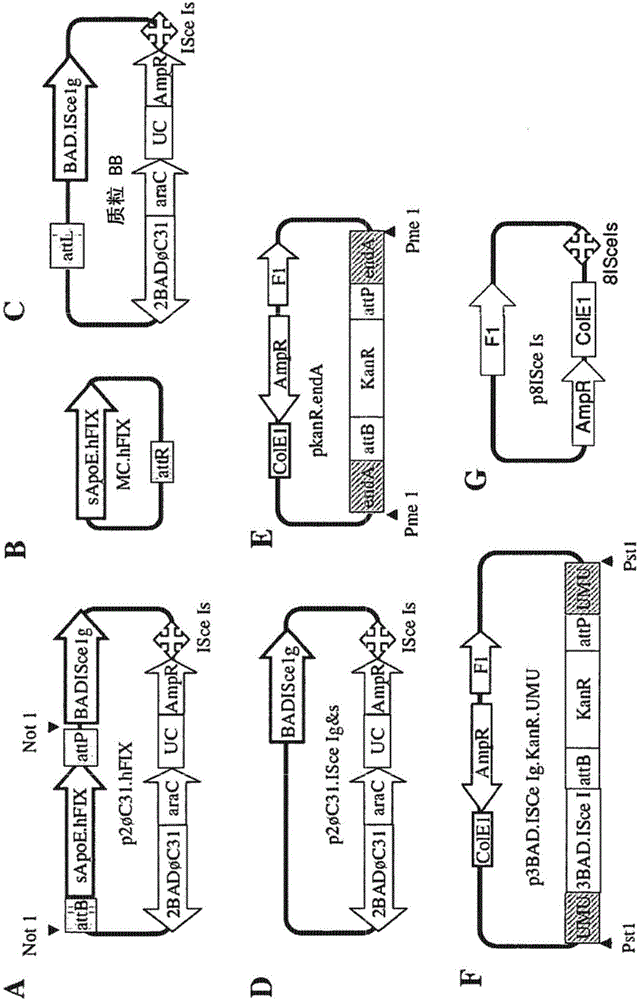
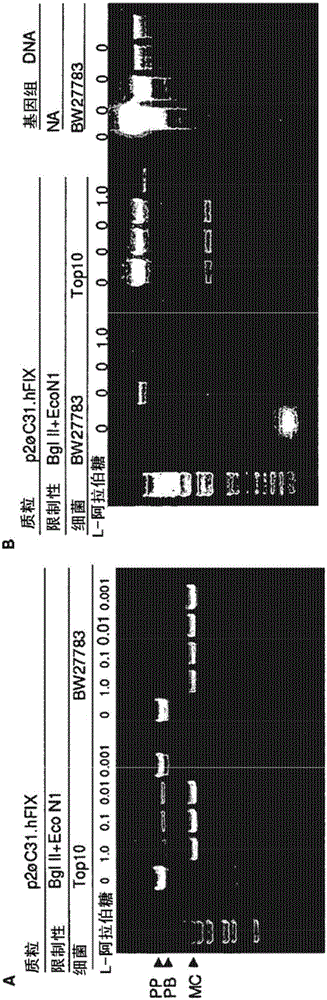
[0140] In our initial protocol, minicircles were used to generate plasmids such as .hFIX( figure 1 A) and Top 10 strains to produce minicircles (Chen ZY et al., Human Gene Therapy 16:126, 2005). In our minicircle preparation, however, we detected small but variable amounts of impurity DNA comprising the unrecombined parental plasmid and the plasmid backbone circle (plasmid BB). We observed that this impurity DNA was mainly due to the "all or nothing" phenomenon: that is, a bacterial subpopulation became unable to express the high load, low affinity L-arabinose transporter araE, and could not absorb L-arabinose and araC -expression under the control of the ABD regulatory system and ISce I gene. Khlebnikov A and colleagues (Microbiology 147:3241, 2001) reported that the constitutive promoter cp8 could be used to drive the expression of araE in strain BW27783 to partially overcome this "all or noth...
Embodiment 2
[0143] Removing the ENDA gene overcomes the DNA degradation problem
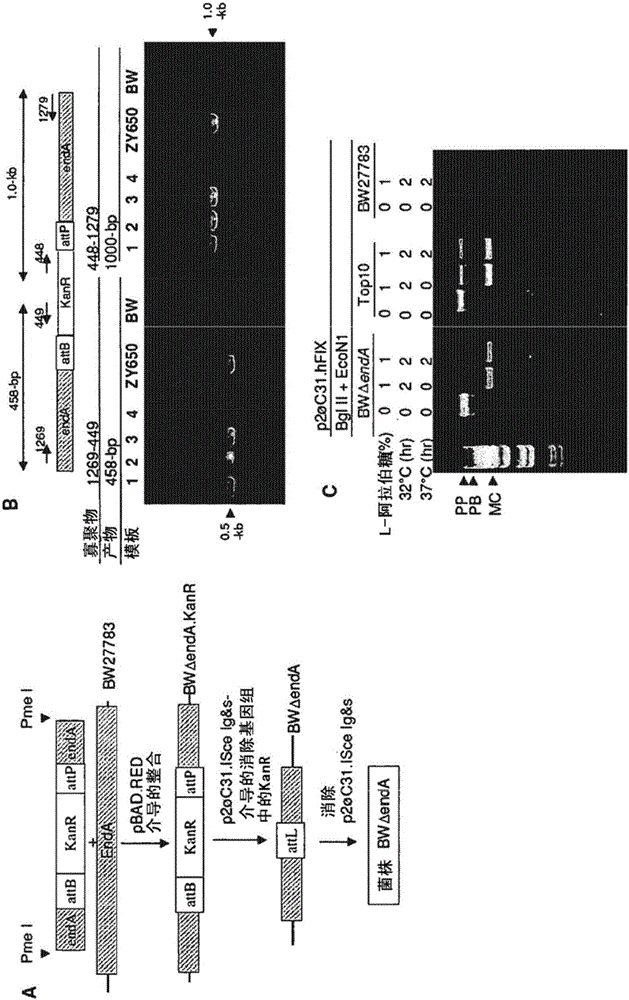
[0144] Since recA is known to affect the stability of plasmids (Khlebnikov A et al., J Bacteriol 182:7029, 2000), we inactivated the recA gene in BW27783 but found this not helpful (data not shown), feeling that this is an endo caused by nuclease 1. We set out to delete the endA gene encoding this DNase. To this end, we generated the plasmid pkanR.endA containing the kanamycin resistance gene flanked by attB and attP sites and two PCR-generated endA gene targeting sequences ( figure 1 E); modified according to the scheme of Datsenko KA and Wanner BL (PNAS 97:6640, 2000), the plasmid was cut with Pme 1 to obtain a linear targeting DNA sequence and integrated into the endA gene of BW27783 ( image 3 A). A first attempt with the proposed protocol using two 35-bp targeting sequences failed (data not shown); however we were successful later by extending the targeting sequences to 329- and 754-bp, respectivel...
Embodiment 3
[0147] Expression of the ISCE I gene in the bacterial genome
[0148] We assume that no - and ISce I - The optimal method for encoding DNA minicircles is to express recombinases from the bacterial genome and restriction enzyme ISce1. We assume that in any culture there are dead bacteria and therefore contamination is unavoidable, these impurity DNAs can cause more harm as they are circular stable, physically indistinguishable from the minicircles and difficult to remove. Instead, put When integrated into the bacterial genome with ISce I, as a linear DNA fragment of the bacterial genome, these harmful genes have less chance of contamination and are easier to remove, and can be degraded by host bacterial exonucleases, which are harmless or have little impact on the recipients. We therefore set out to relocate the BAD.ISce I cassette in the minicircle producing plasmid into the bacterial genome.
[0149] To this end, we generated the plasmid p3BAD.ISCe Ig.KanR.UMU containi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com