A kind of O-acetamide chitosan Schiff base and preparation method thereof
A technology of acetamide chitosan sheet and acetamide chitosan is applied in the field of fine chemical industry, which can solve the problems of inability to meet application requirements, limit the scope of application, etc., and achieve good flocculation and sterilization effects, clear reaction sites, and raw material utilization. high rate effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
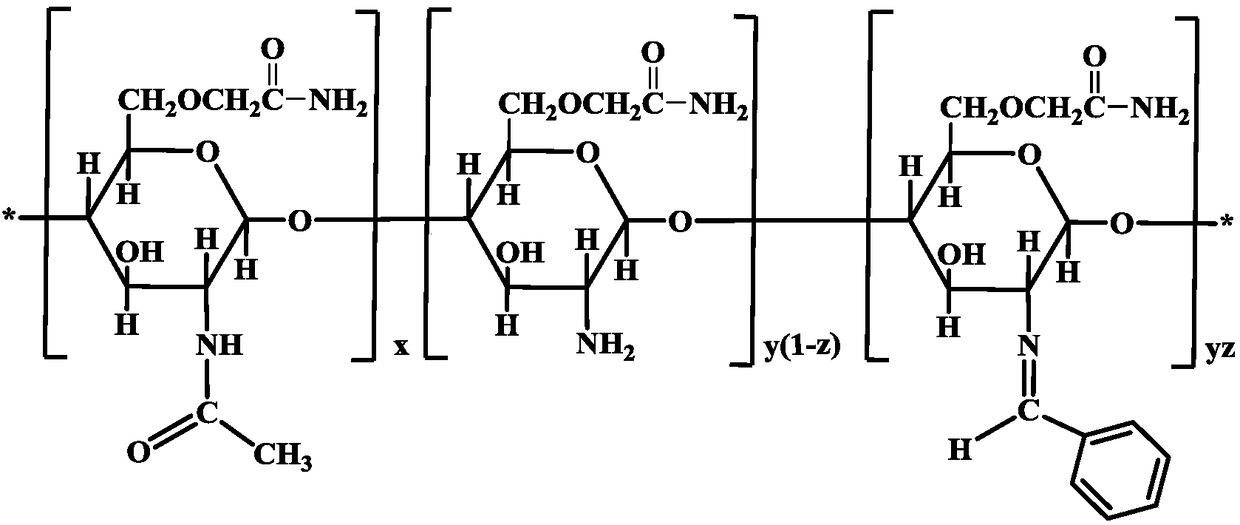
[0048] The preparation process chemical reaction process of O-acetamide chitosan Schiff base of the present invention is as follows figure 1 Shown: First, chitosan and benzaldehyde undergo nucleophilic addition reaction, chitosan on C 2 bit-NH 2 The N atom with a lone electron pair attacks the positively charged C atom on the carbonyl group to complete the nucleophilic addition reaction to form an intermediate product α-hydroxylamine compound, which is further dehydrated to form a Schiff base. The formed Schiff base is further reacted with chloroacetamide for S N 2 Nucleophilic Substitution Reaction, Chitosan C 6 The H atom at the -OH position is replaced by an acetamido group.
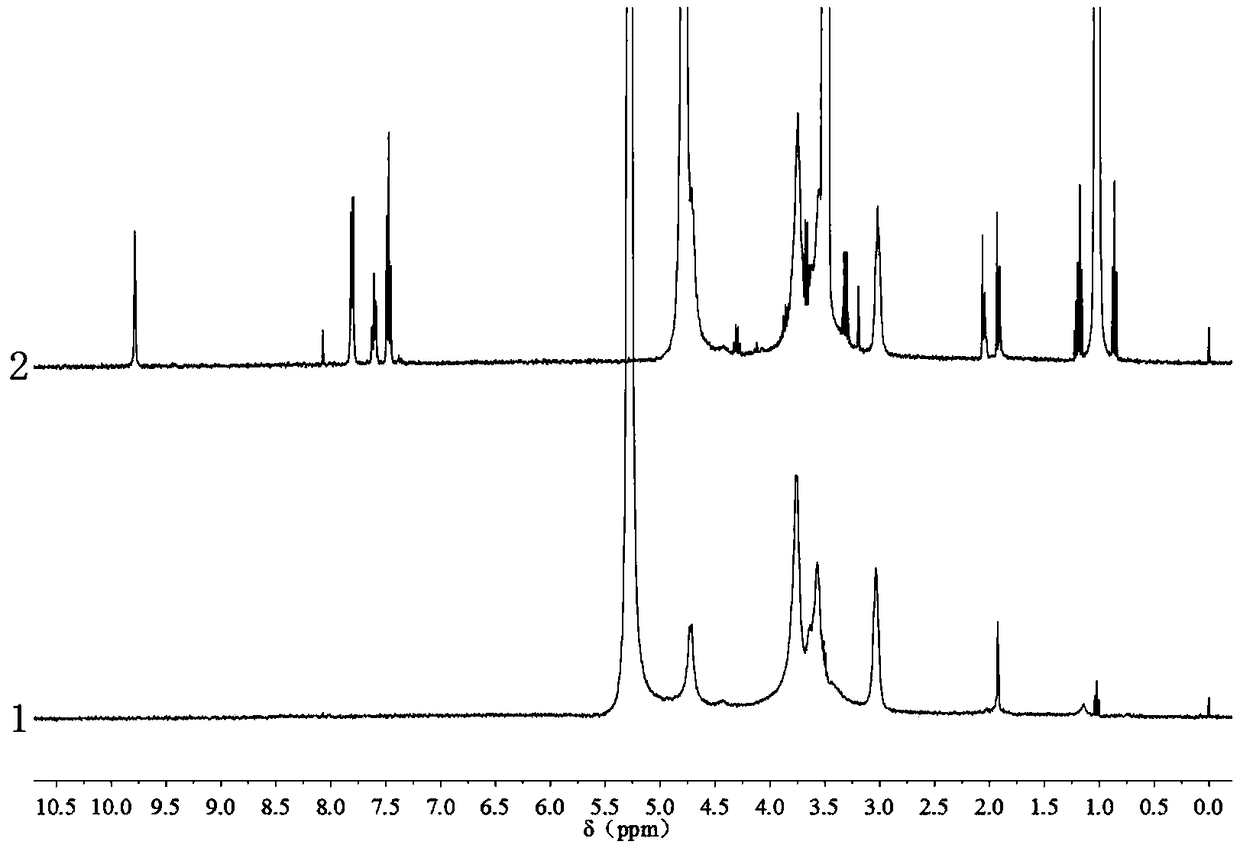
[0049] figure 2 Middle line 1 is the H NMR spectrum of chitosan raw material, and line 2 is the H NMR spectrum of O-acetamide chitosan Schiff base. As can be seen from the figure, in line 1, the multiplet peak at δ=3.4~3.8 belongs to chitosan sugar ring H-3, H-4, H-5, H-6; Single peak assigned to...
Embodiment 1
[0054] A kind of preparation method of O-acetamide chitosan Schiff base, through benzaldehyde chitosan Schiff base and chloroacetamide generation nucleophilic substitution reaction, obtain O-acetamide chitosan Schiff base, its specific process yes:
[0055] Step 1, get 1g chitosan, the molecular weight of chitosan is 700,000, the deacetylation degree is 87%, get 50ml concentration and be 1% acetic acid solution, add chitosan in acetic acid solution, stir 20min at room temperature, The stirring speed is 240r / min until the chitosan is completely dissolved to obtain a solution.
[0056] Step 2, adding 1 mol / L sodium hydroxide solution to the solution, and adjusting the pH of the solution to 7, then performing suction filtration to remove water, and washing with ethanol to obtain filter cake a.
[0057] Step 3, take 50ml of ethanol, add the filter cake a to ethanol, stir for 1h, and raise the temperature to 60°C, then add dropwise 20ml of benzaldehyde ethanol solution and 1.9g of...
Embodiment 2
[0065] A kind of preparation method of O-acetamide chitosan Schiff base, through benzaldehyde chitosan Schiff base and chloroacetamide generation nucleophilic substitution reaction, obtain O-acetamide chitosan Schiff base, its specific process yes:
[0066] Step 1, get 1g chitosan, the molecular weight of chitosan is 700,000, the deacetylation degree is 87%, get 50ml concentration and be 1% acetic acid solution, add chitosan in acetic acid solution, stir 20min at room temperature, The stirring speed is 300r / min until the chitosan is completely dissolved to obtain a solution.
[0067] Step 2, adding 1 mol / L sodium hydroxide solution to the solution, and adjusting the pH of the solution to 7, then performing suction filtration to remove water, and washing with ethanol to obtain filter cake a.
[0068] Step 3, take 50ml of ethanol, add the filter cake a into the ethanol, stir for 1 hour, and raise the temperature to 70°C, then add dropwise 20ml of benzaldehyde ethanol solution a...
PUM
| Property | Measurement | Unit |
|---|---|---|
| degree of grafting | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com