A pla2r-thsd7a fusion protein and its application and kit
A PLA2R-THSD7A, fusion protein technology, applied in the medical field, to achieve the effect of safety and method, increase sensitivity and improve detection rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0071] Preparation of embodiment 1 fusion protein
[0072] The preparation of this fusion protein comprises the following steps:
[0073] a. Trizol method to extract human tissue total RNA;
[0074] b. Prepare cDNA using universal primers;
[0075] c. References, use computer and primer design software to design PCR primers and amplify the full-length fragments of PLA2R and THSD7A respectively;
[0076] d. Linking the two full-length sequences into PLA2R-THSD7A and THSD7A-PLA2R two recombinant gene coding sequences respectively through the additional restriction sites of the primers;
[0077] e. Transfer the above recombinant gene sequence into pET SUMO and pcDNA3.1 expression vectors, and perform prokaryotic and eukaryotic expression respectively;
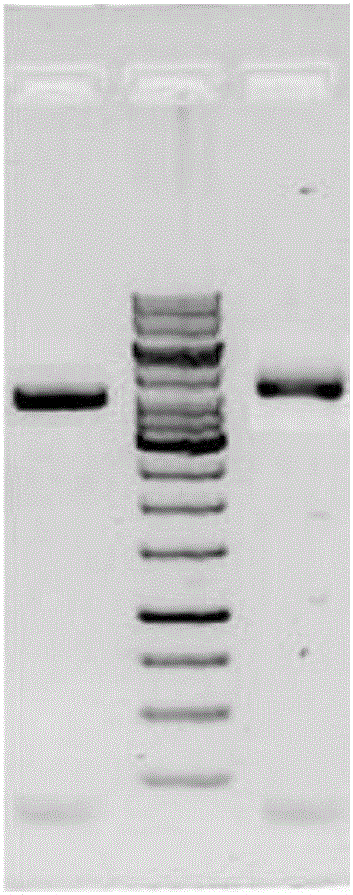
[0078] f. SDS-PAGE electrophoresis and anti-tagged protein antibody WESTERN-BLOT detection of expression products;
[0079] g. Then use the positive antiserum preliminary test to identify;
[0080] h. Amplify, express and pur...
Embodiment 2
[0264] Example 2 Application of PT protein in diagnosis of spontaneous MN
[0265] ELISA method for detecting spontaneous MN-related autoantibodies in subjects using the complex protein PT as an antigen:
[0266] Using PT complex protein to detect spontaneous MN-associated autoantibodies by ELISA method includes: the coating buffer is 0.05M carbonate buffer at pH 9.6 (where Na 2 CO 3 1.59g / L, NaHCO 3 2.93g / L), blocking solution (BSA 10g / L, sucrose 50g / L), washing solution is TBS containing Tween-20, pH7.6 (NaCl 8.7g / L, Tris 2.4g / L), sample diluent (Tris 1.21g / L, BSA 10g / L, Tween-20 0.05%), the chromogenic solution is TMB (3,3",5,5"-tetramethylbenzidine), and the stop solution is 2M sulfuric acid. Secondary antibody diluent (Tris 1.2083g / L, BSA 10g / L, Tween-20 0.05%), positive control and negative control were serum from IMN patients and healthy people from non-IMN patients, respectively.
[0267] The 96-well plate was a Nuc96-well plate, and BSA was purchased from Sigma R...
Embodiment 3
[0285] Embodiment 3 clinical detection application
[0286] The method in Example 2 was used to detect the sera of patients with spontaneous MN, patients with secondary MN, patients with other diseases and normal people, respectively. For 41 cases of clinical spontaneous MN patients, 10 cases of secondary MN patients, 10 cases of other disease patients, and 40 cases of normal human serum detection results are shown in Table 6, Figure 12 .
[0287] At the same time, PLA2R and THSD7A were used as controls.
[0288] Table 6 Comparison of fusion protein of the present invention with PLA2R and THSD7A on test results of subjects
[0289]
[0290] The results show that the detection rate of the fusion protein of the present invention is 78.1% for spontaneous MN patients, and the detection rate for secondary MN patients, patients with other diseases and normal human serum is 0%. The results show that the sensitivity of the fusion protein of the invention to spontaneous MN detec...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com