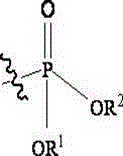
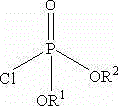
Fosaprepitant derivative, synthesis thereof, and use thereof in long acting preparation
A technology for drugs and compounds, applied in the field of prodrugs and pharmaceutical preparations, can solve problems such as poor patient compliance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0167] Embodiment 1: preparation [3-[[(2R, 3S)-2-[(1R)-1-[3,5-two (trifluoromethyl) phenyl] ethoxy]-3-(4- Fluorophenyl)4-morpholinyl]methyl]-4,5-dihydro-5-oxo-1H-1,2,4-triazol-1-yl]-2-(trimethylammonium)- ethyl phosphate
[0168] Process 1
[0169]
[0170] As shown in Scheme 1 above, choline chloride was reacted with the phosphorylating reagent cyanoethyl N,N-diisopropylamine in anhydrous dichloromethane at room temperature in the presence of N,N-diisopropylethylamine. Propyl chlorophosphoramidite reaction, followed by coupling with aprepitant, followed by oxidation with 30% aqueous hydrogen peroxide gave the crude fosaprepitant derivative in a one-step process. The cyanoethyl protecting group was cleaved with ammonium hydroxide in methanol at room temperature. The resulting phosphodiester was further converted to the corresponding ammonium phosphate zwitterion salt by reaction with aqueous sodium hydroxide (0.1M) in methanol.
Embodiment 2
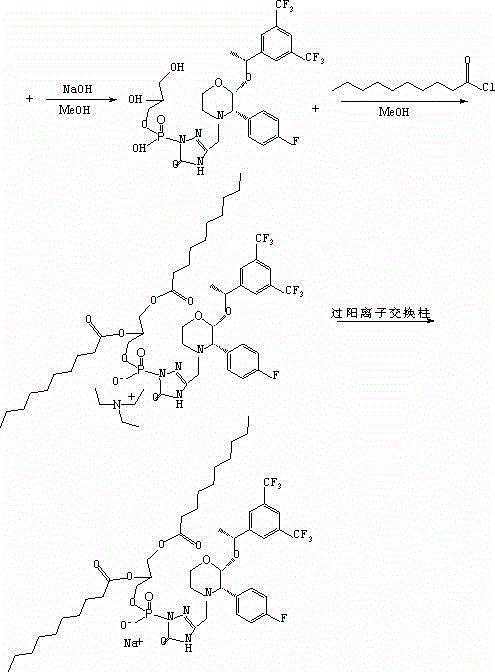
[0171] Embodiment 2: Preparation of 2-(decanoyloxy) ethyl-[3-[[(2R, 3S)-2-[(1R)-1-[3,5-bis(trifluoromethyl)phenyl ]ethoxy]-3-(4-fluorophenyl)4-morpholinyl]methyl]-4,5-dihydro-5-oxo-1H-1,2,4-triazole-1- base] sodium phosphate
[0172] Process 2
[0173]
[0174]
[0175] As shown in Scheme 2 above, the reaction between 1-decanoyl chloride and ethylene glycol in anhydrous dichloromethane at room temperature in the presence of triethylamine affords the hydroxy ester. At room temperature, in anhydrous dichloromethane, in the presence of N, N-diisopropylethylamine, react with the phosphorylating reagent cyanoethyl N, N-diisopropylphosphoramidite chloride, followed by reaction with Aprepitant was coupled, followed by oxidation with 30% aqueous hydrogen peroxide to obtain fosaprepitant derivatives in a one-step process. The cyanoethyl protecting group was cleaved with ammonium hydroxide in methanol at room temperature. The resulting phosphodiester was further saponified by ...
Embodiment 3
[0176] Embodiment 3: Preparation [3-[[(2R, 3S)-2-[(1R)-1-[3,5-bis(trifluoromethyl)phenyl]ethoxy]-3-(4- Fluorophenyl)4-morpholinyl]methyl]-4,5-dihydro-5-oxo-1H-1,2,4-triazol-1-yl]-2-(nonanyloxy) Sodium ethyl phosphate
[0177] Process 3
[0178]
[0179] As shown in Scheme 3 above, the reaction between 1-nonanol and ethylene glycol in refluxing toluene in the presence of p-toluenesulfonic acid gives the hydroxy ether. The resulting hydroxyl ether was reacted with the phosphorylating reagent cyanoethyl N,N-diisopropylphosphoramidite chloride in the presence of N,N-diisopropylethylamine in anhydrous dichloromethane at room temperature The reaction, followed by coupling with aprepitant, followed by oxidation with 30% aqueous hydrogen peroxide gave the fosaprepitant derivative in a one-step process. The cyanoethyl protecting group was cleaved with ammonium hydroxide in methanol at room temperature and the resulting phosphodiester was converted to the sodium salt by reaction w...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com