Method for preparing medicine ceftriaxone sodium crystal compound for treating surgical infection
A technology for ceftriaxone sodium and a crystal compound, which is applied in the field of medicine and achieves the effects of high purity, simple process and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044] The preparation of embodiment 1 ceftriaxone sodium crystal compound
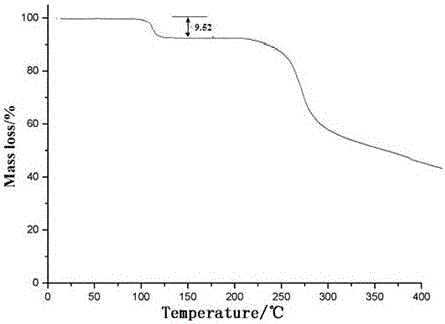
[0045] Dissolve ceftriaxone sodium in a mixed solvent of water and dimethyl sulfoxide whose volume is 4 times the weight of ceftriaxone sodium at 35°C. The volume ratio of water to dimethyl sulfoxide is 5:1.5; Add the mixed solvent of ethanol and ether whose volume is 7 times of the weight of ceftriaxone sodium at a speed of 2:2, stir while adding, control the temperature at 35°C, and grow the crystal for 3 hours; then Add isopropanol with a volume 5 times the weight of ceftriaxone sodium at a speed of 20ml / min, and after growing the crystal for 1 hour, cool down to -5°C at a speed of 10°C / hour, then keep stirring at a speed of 90 rev / min and stir to analyze crystallization and crystal growth for 3 hours; filtering, washing, and drying under reduced pressure to obtain ceftriaxone sodium crystalline compound.
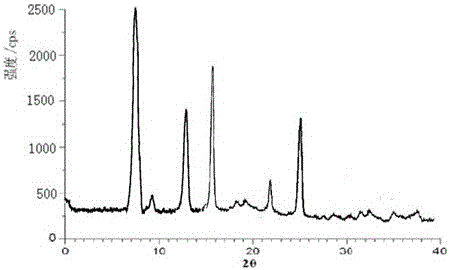
[0046] Measured by powder X-ray diffractometry, the X-ray powder diffraction pattern repres...
Embodiment 2
[0049] The preparation of embodiment 2 ceftriaxone sodium crystalline compound
[0050] Dissolve ceftriaxone sodium in a mixed solvent of water and dimethyl sulfoxide whose volume is 5 times the weight of ceftriaxone sodium at 35°C. The volume ratio of water to dimethyl sulfoxide is 5:1.5; Add the mixed solvent of ethanol and ether whose volume is 8 times of the weight of ceftriaxone sodium at a speed of 2:2, stir while adding, control the temperature at 35°C, and grow the crystal for 3 hours; then Add isopropanol with a volume of 6 times the weight of ceftriaxone sodium at a speed of 20ml / min, and after growing the crystal for 1 hour, cool down to -5°C at a speed of 10°C / hour, and then maintain a stirring speed of 90 rpm to stir and analyze crystallization and crystal growth for 3 hours; filtering, washing, and drying under reduced pressure to obtain ceftriaxone sodium crystalline compound.
[0051] Measure with powder X-ray diffractometry, the X-ray powder diffraction patte...
Embodiment 3
[0052] The preparation of embodiment 3 ceftriaxone sodium crystalline compound
[0053] Dissolve ceftriaxone sodium in a mixed solvent of water and dimethyl sulfoxide whose volume is 6 times the weight of ceftriaxone sodium at 35°C. The volume ratio of water to dimethyl sulfoxide is 5:1.5; Add the mixed solvent of ethanol and diethyl ether whose volume is 9 times of the weight of ceftriaxone sodium at a high speed, the volume ratio of ethanol and diethyl ether is 2:2, stir while adding, control the temperature at 35°C, and grow crystals for 3 hours; then Add isopropanol whose volume is 7 times the weight of ceftriaxone sodium at a speed of 20ml / min, and after growing the crystal for 1 hour, cool down to -5°C at a speed of 10°C / hour, then keep stirring at a speed of 90 rev / min and stir to analyze crystallization and crystal growth for 3 hours; filtering, washing, and drying under reduced pressure to obtain ceftriaxone sodium crystalline compound.
[0054] Measure with powder X...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com