Injection cefoperazone sodium and sulbactam sodium pharmaceutical composition for reducing allergic reaction
A technology of cefoperazone sodium sulbactam sodium and cefoperazone sodium, which is applied in the compound pharmaceutical composition of cefoperazone sodium sulbactam sodium for injection and its preparation field, can solve the problems such as weak antibacterial activity, and achieve stable product quality, related substances and The content change range is small, and the product solubility is good
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
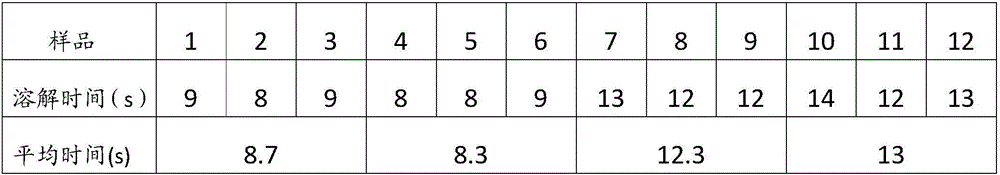
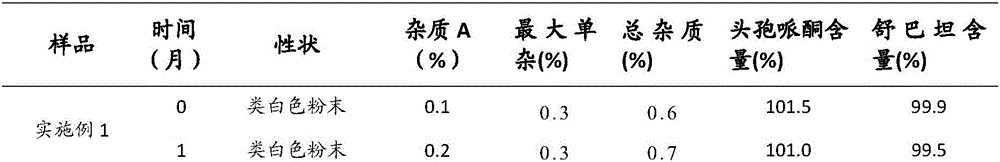
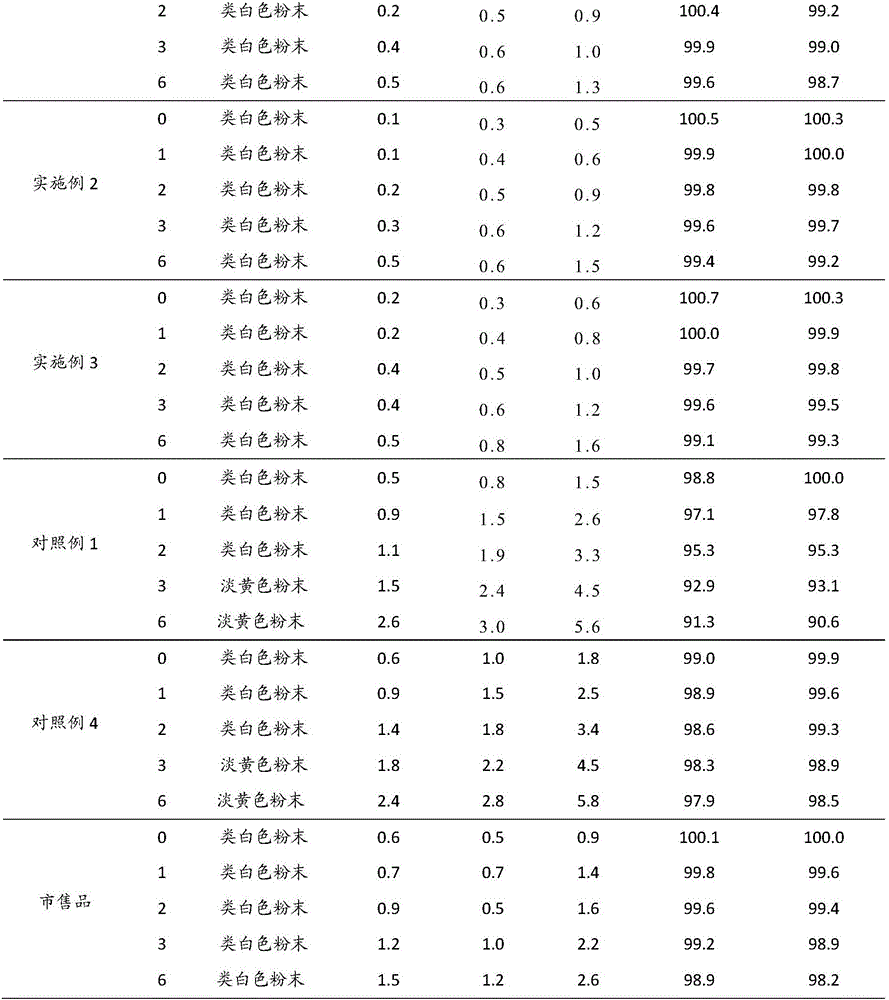
[0037] Cefoperazone Sodium Sulbactam Sodium for Injection (specification: 0.75g, calculated as available acid, 1000 bottles) and preparation:
[0038] Cefoperazone sodium (calculated as cefoperazone) 500g
[0039] Sulbactam sodium (as sulbactam) 250g
[0040] Acetic acid-sodium acetate appropriate amount
[0041] Weigh the prescribed amount of cefoperazone sodium and sulbactam sodium, dissolve them in 1200ml of water for injection, adjust the pH value of the solution to 5.6-5.8 with acetic acid-sodium acetate, add 300ml of water for injection, and add activated carbon for injection to absorb the heat source. 0.45μm and 0.22μm pore size membrane filters are sterile filtered, and the sterile solution is put into a bottle and freeze-dried.
Embodiment 2
[0049] Cefoperazone Sodium and Sulbactam Sodium for Injection (specification: 1.5g, calculated as available acid, 1000 bottles) and preparation:
[0050] Cefoperazone sodium (calculated as cefoperazone) 750g
[0051] Sulbactam sodium (as sulbactam) 750g
[0052] Acetic acid-sodium acetate appropriate amount
[0053] Weigh the prescribed amounts of cefoperazone sodium and sulbactam sodium, dissolve in 3600ml of water for injection, adjust the pH of the solution to 5.4-5.6 with acetic acid-sodium acetate, add 900ml of water for injection, and add activated carbon for injection to absorb the heat source. 0.45μm and 0.22μm pore size membrane filters are sterile filtered, and the sterile solution is put into a bottle and freeze-dried.
Embodiment 3
[0061] Cefoperazone Sodium Sulbactam Sodium for Injection (specification: 3.0g, calculated as available acid, 1000 bottles) and preparation:
[0062] Cefoperazone sodium (calculated as cefoperazone) 1500g
[0063] Sulbactam sodium (as sulbactam) 1500g
[0064] Acetic acid-sodium acetate appropriate amount
[0065] Weigh the prescribed amount of cefoperazone sodium and sulbactam sodium, dissolve in 9600ml of water for injection, adjust the pH value of the solution to 5.6-5.8 with acetic acid-sodium acetate, add 2400ml of water for injection, add activated carbon for injection to absorb the heat source, and use 0.45 It is obtained by sterilizing filtration through membrane filters with pore diameters of μm and 0.22 μm, filling the sterile solution into a bottle, and lyophilizing it.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com