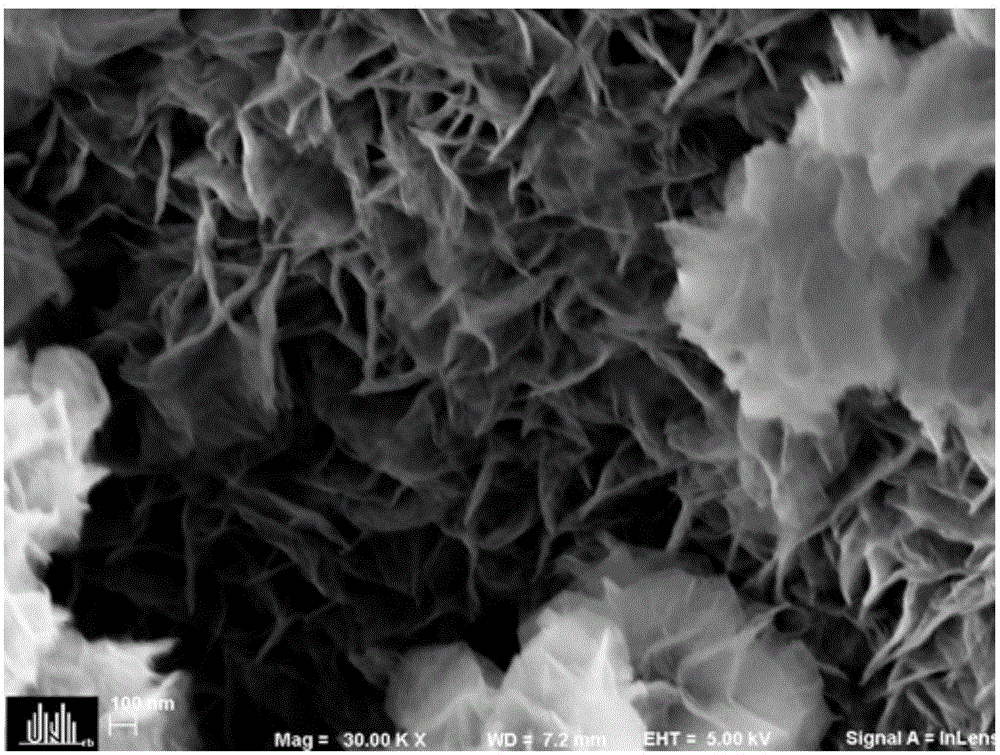
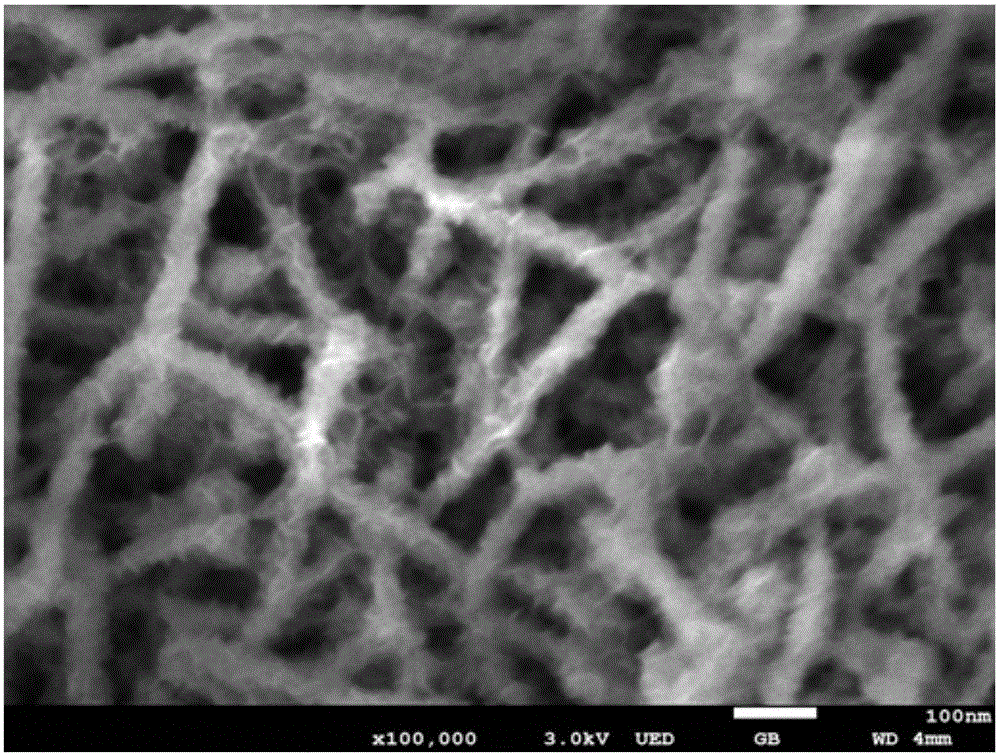
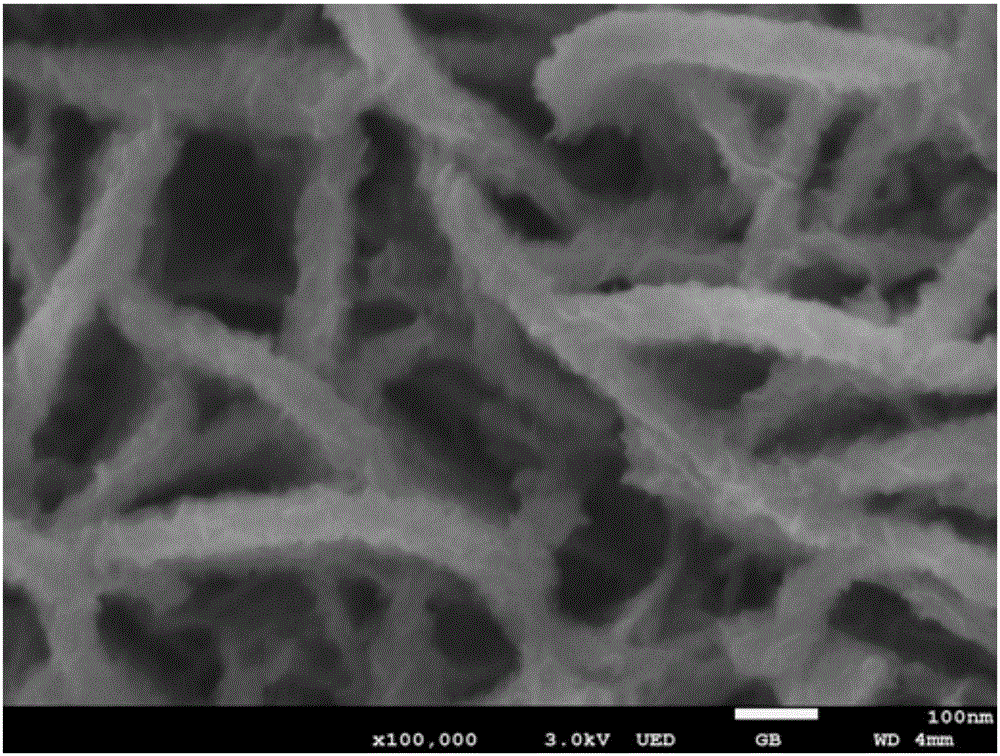
Preparation method of layered ferronickel hydroxide electrode
A hydroxide, nickel-iron technology, applied in electrodes, electrolysis process, electrolysis components, etc., can solve the problem of poor chemical stability and activity of layered nickel-iron hydroxide, and limit the industrial utilization of layered nickel-iron hydroxide, etc. problems, to achieve the effect of superior electrocatalytic activity and stability, good sample activity, and strong controllability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] 1) Put 2cm×5cm copper foam in concentrated hydrochloric acid for ultrasonic cleaning for 5 minutes, rinse the foam copper repeatedly with deionized water, and then ultrasonically clean it in deionized water for 5 minutes;
[0035] 2) Prepare a solution containing iron salt, nickel salt and urea, wherein the concentration of iron salt is: 18mmol / L, the concentration of nickel salt is: 12mmol / L, and the concentration of urea is: 60mmol / L; wherein, the iron salt is ferric sulfate; The nickel salt is nickel sulfate.
[0036] 3) Take an appropriate amount of the above solution and put it in a 30mL stainless steel reactor with Teflon lining and put it into the copper foam cleaned in step 1). The hydrothermal kettle was placed in an oven at 120°C for 12 hours, cooled to room temperature naturally, rinsed repeatedly with deionized water and absolute ethanol, and washed with N 2 Perform purging and drying to obtain a NiFe-LDH electrode; wherein, the volume filling amount of the...
Embodiment 2
[0041] 1) Put 2cm×5cm nickel foam in concentrated hydrochloric acid for ultrasonic cleaning for 5 minutes, rinse the foam nickel repeatedly with deionized water, and then put it into deionized water for ultrasonic cleaning for 5 minutes;
[0042] 2) Prepare a solution containing iron salt, nickel salt and urea, wherein the concentration of iron salt is: 18mmol / L, the concentration of nickel salt is: 12mmol / L, and the concentration of urea is: 60mmol / L; wherein, the iron salt is ferric chloride ; The nickel salt is nickel chloride.
[0043] 3) Take an appropriate amount of the above solution and put it in a 30mL stainless steel reaction kettle with a polytetrafluoroethylene lining and put it into the nickel foam cleaned in step 1). The hydrothermal kettle was placed in an oven at 120°C for 12 hours, cooled to room temperature naturally, rinsed repeatedly with deionized water and absolute ethanol, and washed with N 2 Perform purging and drying to obtain a NiFe-LDH electrode; wh...
Embodiment 3
[0047] 1) Put the 2cm×5cm copper foam in concentrated hydrochloric acid for ultrasonic cleaning for 5 minutes, rinse the foam copper repeatedly with deionized water, and then put it into deionized water for ultrasonic cleaning for 5 minutes;
[0048] 2) Prepare a solution containing iron salt, nickel salt and urea, wherein the concentration of iron salt is: 18mmol / L, the concentration of nickel salt is: 12mmol / L, and the concentration of urea is: 60mmol / L; wherein, the iron salt is iron acetate; The nickel salt is nickel acetate.
[0049] 3) Take an appropriate amount of the above solution and put it in a 30mL stainless steel reactor with Teflon lining and put it into the copper foam cleaned in step 1). The hydrothermal kettle was placed in an oven at 120°C for 12 hours, cooled to room temperature naturally, rinsed repeatedly with deionized water and absolute ethanol, and washed with N 2 Perform purging and drying to obtain a NiFe-LDH electrode; wherein, the volume filling am...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com