A kind of hydrogel with micro-current and sustained drug release, its preparation method and application
A micro-current and hydrogel technology, applied in electrotherapy, magnetic field generated by permanent magnets, magnetic therapy, etc., can solve the problem of drug slow release ability and healing promotion ability, poor mechanical properties of hydrogel, wound surface No protective effect and other problems, to achieve good electron transport ability, improve mechanical properties, and enrich the effect of surface defects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
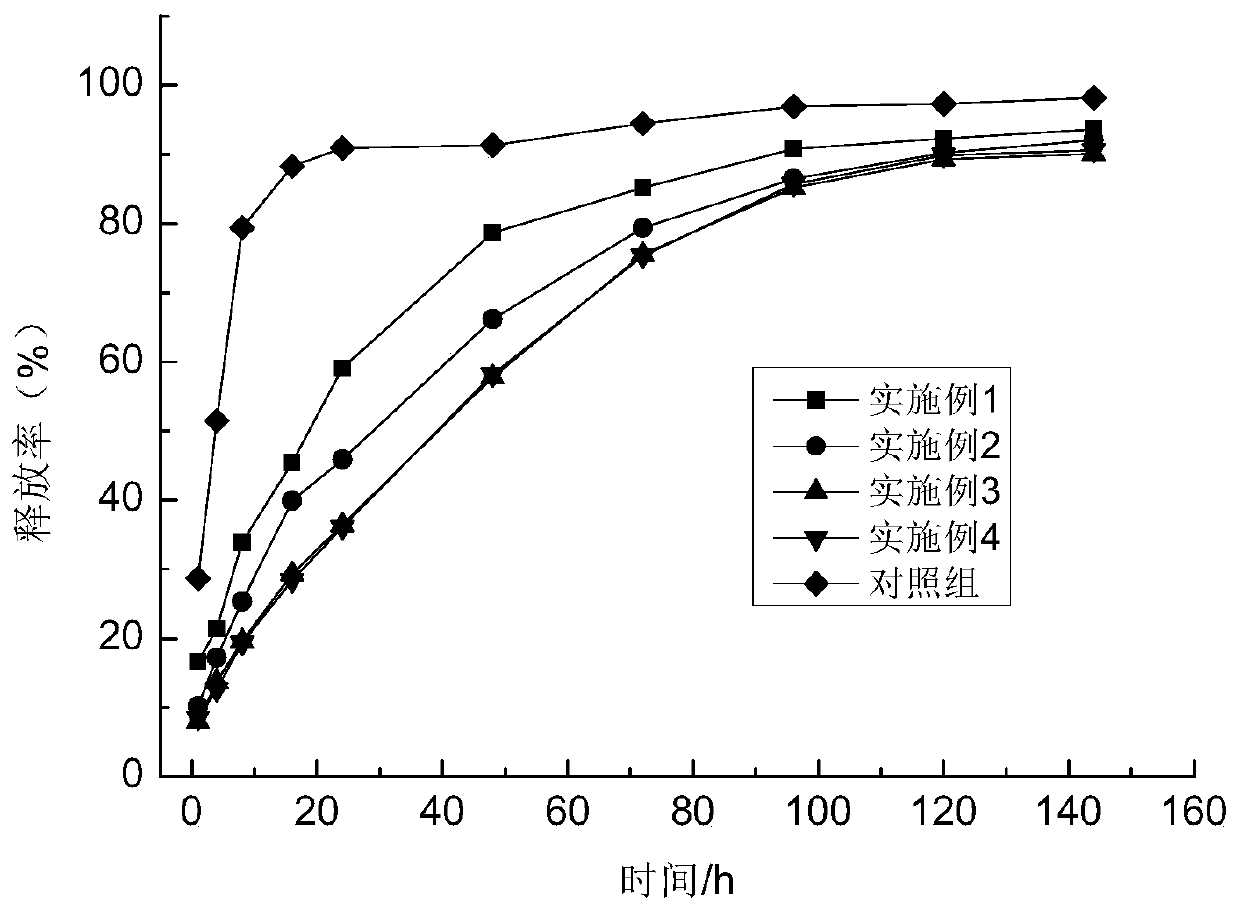
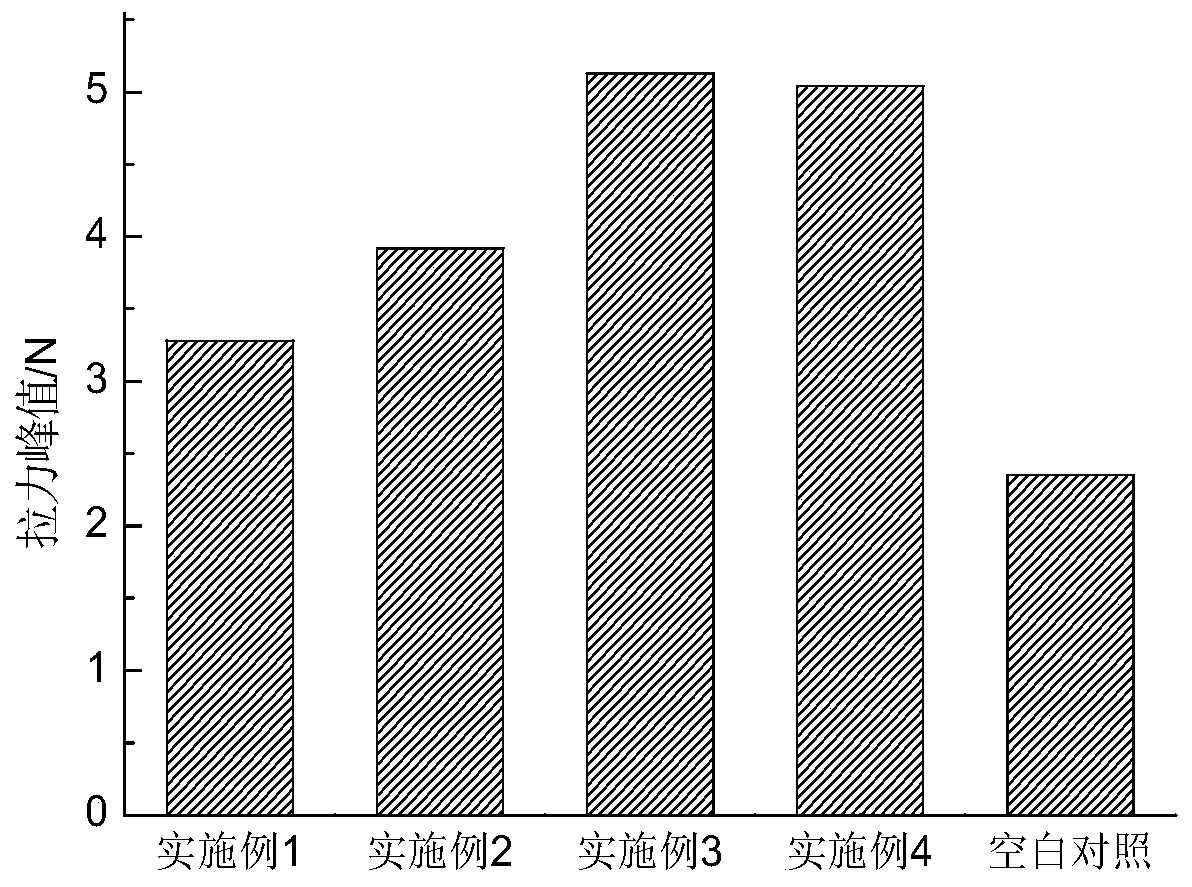
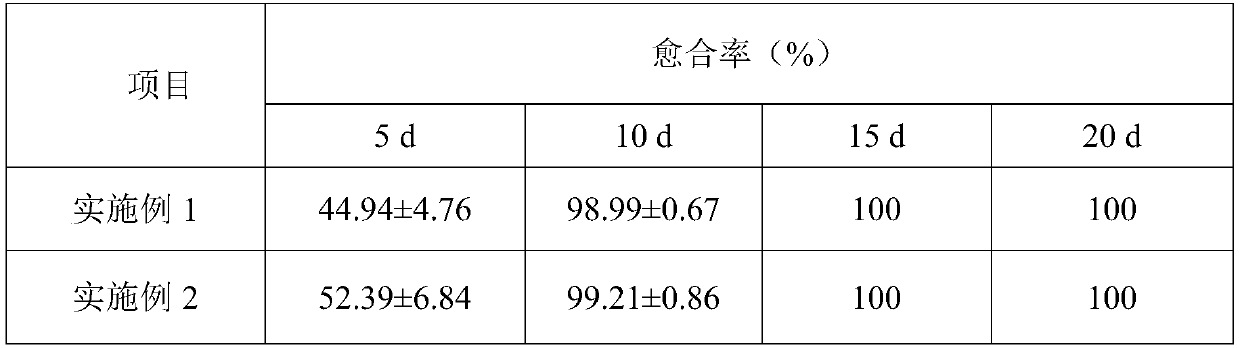
Examples
Embodiment 1
[0049] A hydrogel with micro-current and drug sustained release, the specific preparation method is as follows:
[0050] (1) Dissolve 0.5g epidermal growth factor in 50mL deionized water, add 25g carbon quantum dots, stir magnetically at 30°C and 40rpm for 1h, centrifuge the product at 4000rpm for 10min, discard the supernatant, and The residue was washed successively with ethanol and deionized water, repeated three times, and finally freeze-dried for 24 hours to obtain drug-loaded carbon quantum dots.
[0051] (2) Dissolve 1g of sodium alginate powder and 1g of sodium carboxymethylcellulose powder in 100mL of deionized water respectively. After dissolving, add 1g of polyvinylidene fluoride powder and 1g of permanent magnet nano Granules (magnetic field strength is 0.15 tesla), 1g of drug-loaded carbon quantum dots obtained in step (1) and 3g of anhydrous calcium carbonate powder, after stirring evenly, add carboxymethylcellulose sodium solution.
[0052] (3) Add 6g of glucon...
Embodiment 2
[0054] A hydrogel with micro-current and drug sustained release, the specific preparation method is as follows:
[0055] (1) Dissolve 0.5g of epidermal growth factor in 50mL of deionized water, then add 15g of carbon quantum dots, stir magnetically at 37°C and 40rpm for 2h, centrifuge the product at 4000rpm for 10min, and use ethanol, deionized Water was washed sequentially, repeated 3 times, and finally freeze-dried for 24 hours to obtain drug-loaded carbon quantum dots.
[0056] (2) Dissolve 1.5g of sodium alginate powder and 1.5g of sodium carboxymethylcellulose powder in 100mL of deionized water respectively. After dissolving, add 1.2g of polyvinylidene fluoride powder, 4.8 g permanent magnet nanoparticles (magnetic field strength is 0.25 tesla), 1.5 g of drug-loaded carbon quantum dots obtained in step (1) and 6 g of anhydrous calcium carbonate powder, after stirring evenly, add carboxymethyl cellulose sodium solution.
[0057] (3) Finally, add 12g of gluconolactone, sti...
Embodiment 3
[0059] A hydrogel with micro-current and drug sustained release, the specific preparation method is as follows:
[0060] (1) Dissolve 0.5g of epidermal growth factor in 50mL of deionized water, then add 2.5g of carbon quantum dots, stir magnetically at 45°C and 40rpm for 3h, centrifuge the product at 4000rpm for 10min, and use ethanol, deionized Ionized water was washed sequentially, repeated 3 times, and finally freeze-dried for 24 hours to obtain drug-loaded carbon quantum dots.
[0061] (2) Dissolve 2g of sodium alginate powder and 2g of sodium carboxymethyl cellulose powder in 100mL of deionized water respectively. After dissolving, add 1.67g of polyvinylidene fluoride powder, 8.33g of yam Magnet nanoparticles (the particle magnetic field strength is 0.35 Tesla), 2g of the drug-loaded carbon quantum dots obtained in step (1) and 10g of anhydrous calcium carbonate powder are stirred evenly, and then sodium carboxymethylcellulose solution is added.
[0062] (3) Finally, 20 ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| size | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com