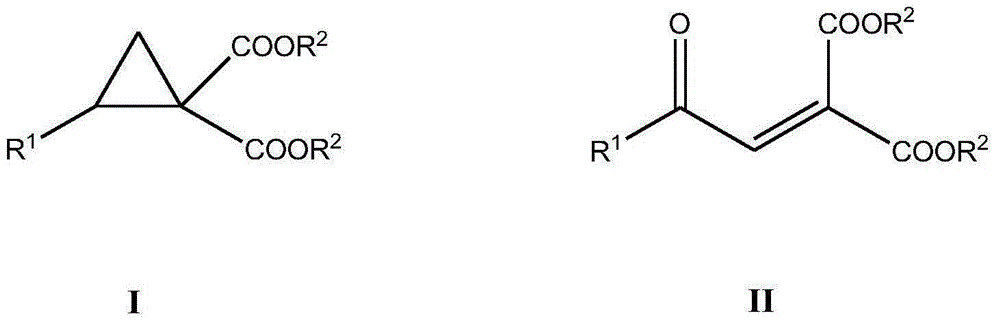
Method for synthesizing benzoyl methylene malonic ester compound
A technology of benzoylmethylene malonate and synthesis method, which is applied to the preparation of organic compounds, chemical instruments and methods, and preparation of carboxylate, can solve the problems of difficult synthesis and industrial application limitations, and achieve Easy operation, low cost, cheap and easy-to-obtain catalyst
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030]
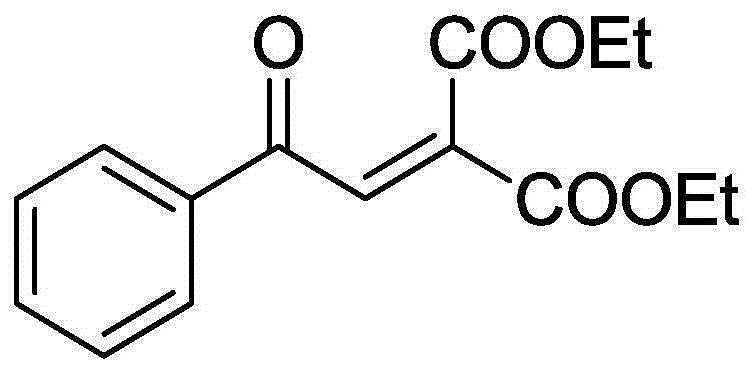
[0031] With 52.4mg (0.2mmol) 2-phenyl-1,1-dicarboxycyclopropane, 1.63mg (0.01mmol) FeCl 3 , 141.7mg (0.4mmol) Selectfluor, was added to a 10mL round bottom flask, and then 4mL of acetonitrile was added as a solvent. Then, magnetically stir at 80° C. for 12 h. Then, 1g of column chromatography silica gel (100-200 mesh) was added to the reaction solution, and the solvent was removed by distillation under reduced pressure. : 1) as eluent, collect the eluent that contains product, and eluent evaporates solvent and obtains product pure product benzoylmethylenemalonate ethyl ester. The material was a yellow liquid in 79% yield.
[0032] Characterization data: 1 H NMR (CDCl 3 , 400MHz): δ7.96(d, J=8.5Hz, 2H), 7.84(s, 1H), 7.64-7.61(m, 1H), 7.54-7.49(m, 2H), 4.37-4.27(m, 4H ), 1.35(t, J=7.0Hz, 3H), 1.26(t, J=7.0Hz, 3H); 13 CNMR (100MHz, CDCl 3 ): δ189.3, 164.5, 162.9, 136.6, 136.2, 135.3, 134.2, 129.0, 128.9, 62.5, 62.0, 14.1, 13.7.
Embodiment 2
[0034]
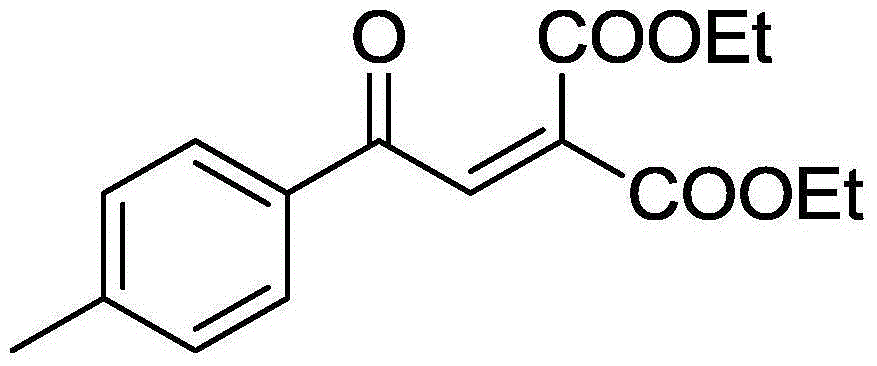
[0035] 55.2 mg (0.2 mmol) of 2-(4-methylphenyl)-1,1-dicarboethoxycyclopropane, 3.25 mg (0.02 mmol) of FeCl 3 , 141.7mg (0.4mmol) Selectfluor, was added to a 10mL round bottom flask, and then 4mL of acetonitrile was added as a solvent. Then, magnetic stirring was performed at 70° C. for 16 h. Then, 1g of column chromatography silica gel (100-200 mesh) was added to the reaction solution, and the solvent was removed by distillation under reduced pressure. : 1) as eluent, collect the eluent containing product, the eluent evaporates solvent to obtain product pure product ethyl 4-methylbenzoylmethylenemalonate. The material was a yellow liquid, 73% yield.
[0036] Characterization data: 1 H NMR (CDCl 3 , 400MHz): δ7.87-7.83(m, 3H), 7.30(d, J=8.0Hz, 2H), 4.37-4.25(m, 4H), 2.43(s, 3H), 1.35(t, J=7.2 Hz, 3H), 1.26(t, J=7.0Hz, 3H); 13 C NMR (100MHz, CDCl 3 ): δ188.8, 164.6, 163.1, 145.5, 136.2, 135.6, 133.7, 129.7, 129.0, 62.4, 61.9, 21.8, 14.0, 13.8.
Embodiment 3
[0038]
[0039] 67.2mg (0.2mmol) of 2-(3,4-dimethoxyphenyl)-1,1-diethylcarboxycyclopropane, 1.63mg (0.01mmol) of FeCl 3 , 70.8mg (0.2mmol) Selectfluor, was added to a 10mL round bottom flask, and then 3mL of acetonitrile was added as a solvent. Then, magnetic stirring was performed at 90° C. for 12 h. Then, 1g of column chromatography silica gel (100-200 mesh) was added to the reaction solution, and the solvent was removed by distillation under reduced pressure. : 1) As an eluent, collect the eluent containing the product, and the eluent is evaporated to remove the solvent to obtain the pure product 3,4-dimethoxybenzoylmethylenemalonate ethyl ester. The material was a yellow liquid, 57% yield.
[0040] Characterization data: 1 H NMR (CDCl 3 , 400MHz): δ7.85(s, 1H), 7.61(d, J=10.4Hz, 1H), 7.55(s, 1H), 6.93(d, J=8.4Hz, 1H), 4.37-4.29(m, 4H), 3.98(s, 3H), 3.94(s, 3H), 1.37(t, J=7.2Hz, 3H), 1.28(t, J=7.0Hz, 3H); 13 C NMR (100MHz, CDCl3): δ187.4, 164.8, 163.1, 154.5, 149.7, ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com