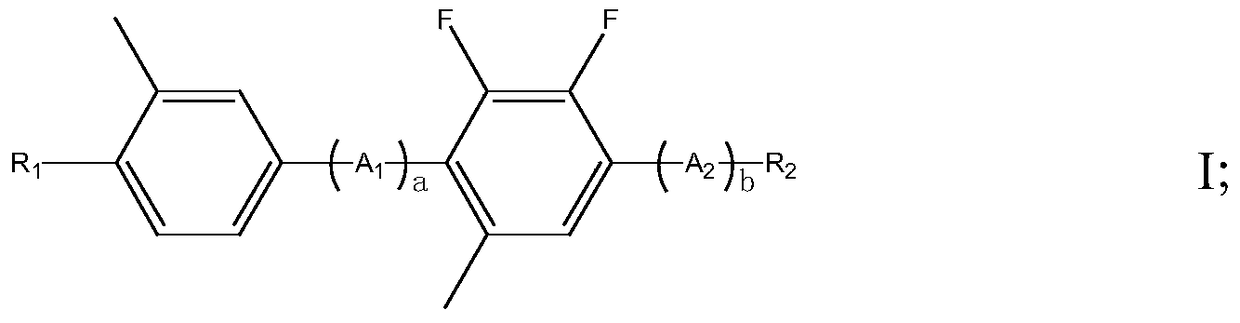
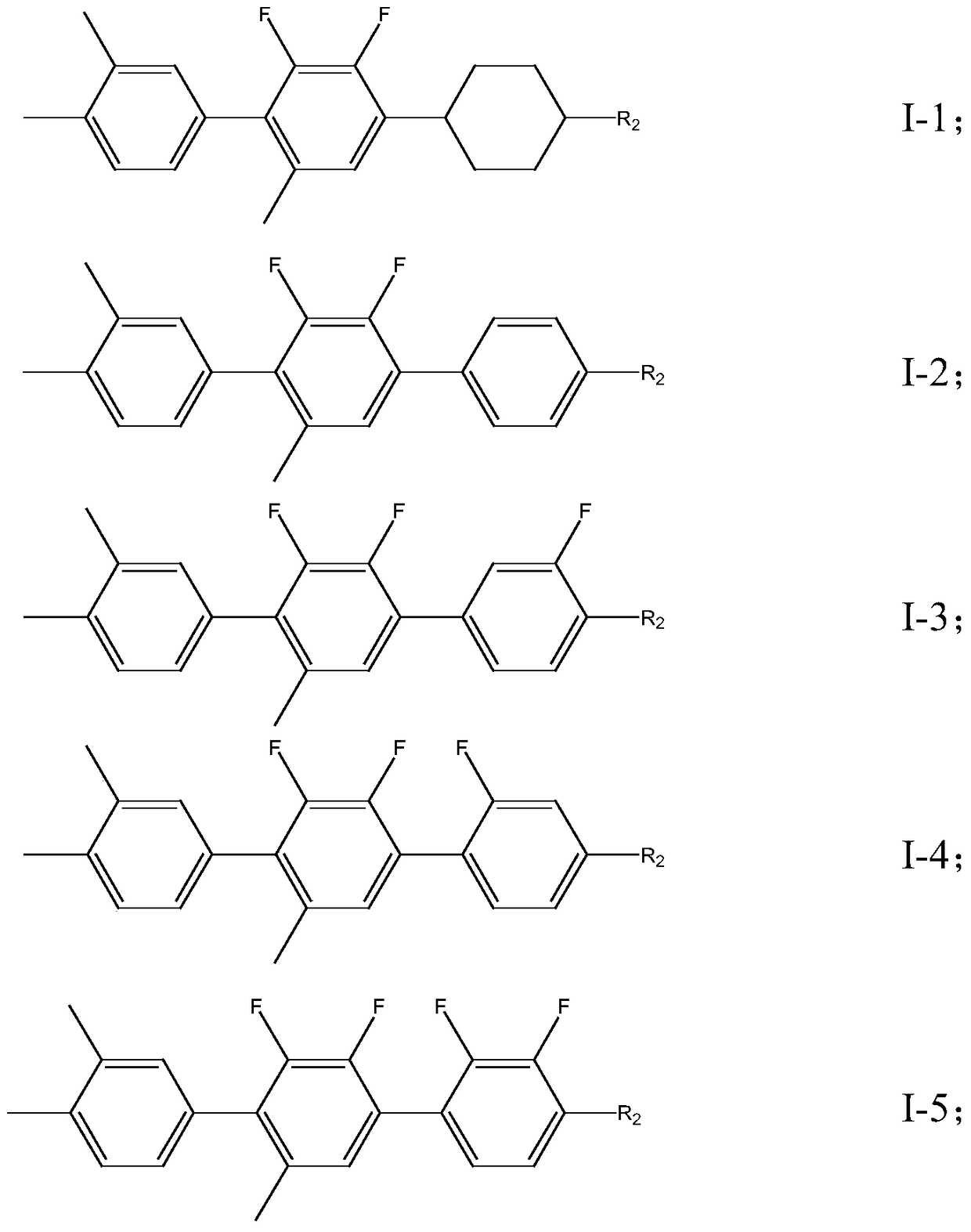
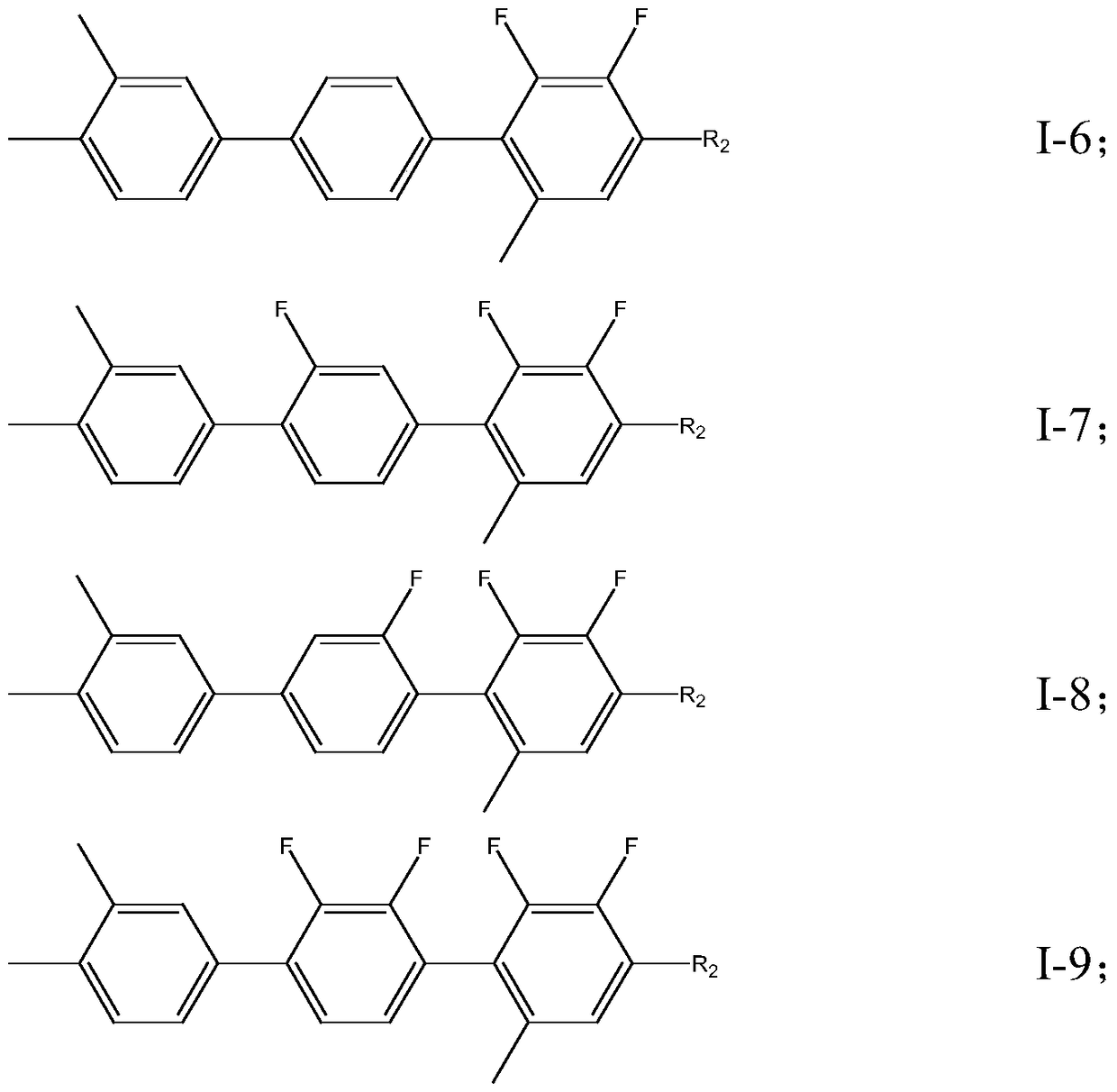
A liquid crystal compound containing 2,3-difluoro-5-methylphenyl, composition and application thereof
A liquid crystal compound, methyl phenyl technology, applied in the preparation of organic compounds, organic chemistry, liquid crystal materials, etc., can solve the problems of insufficient voltage, TFT-LCD response is not fast enough, charge retention rate is not high enough, etc., to achieve stable performance , broad application prospects, large optical anisotropy effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0052] 1. Liquid crystal compound LC-01 containing 2,3-difluoro-5-methylphenyl unit, the structure is as follows:
[0053]
[0054] 2. Synthesize the compound according to the following routes and steps:
[0055]
[0056] The specific steps are:
[0057] (1) Synthesis of 2,3-difluoro-5-methylbenzeneboronic acid (compound II-7):
[0058] Add 12.8g 3,4-difluorotoluene (0.1mol) and 500ml tetrahydrofuran to a 1L dry and clean three-neck flask, protect with nitrogen, cool down to -75℃~-85℃ with liquid nitrogen, add 40ml butyllithium dropwise, and control Warm reaction for 1 hour, dropwise add 15.6g of trimethyl borate (0.15mol), after dropping, control the temperature at -75°C to -85°C for 30 minutes, then naturally raise the temperature to -20°C, add dropwise 200ml of hydrochloric acid aqueous solution; The phase was extracted twice with 100ml×2 ethyl acetate, the organic phases were combined, and spin-dried to obtain 15.5g of a white solid; the liquid phase purity of the ...
Embodiment 2
[0067] 1. Liquid crystal compound LC-02 containing 2,3-difluoro-5-methylphenyl unit, the structure is as follows:
[0068]
[0069] 2. Synthesize the compound according to the following routes and steps:
[0070]
[0071] The specific steps are:
[0072] (1) Synthesis of 3,4-dimethylphenylboronic acid (compound 2-4):
[0073] Add 18.5g of 3,4-difluorobromobenzene (ie 0.1mol of compound 2-3) and 500ml of tetrahydrofuran into a 1L dry and clean three-necked flask, react with 2.64g of magnesium chips (0.11mol) under nitrogen protection, reflux for 1h, and then cool down To -30-~-40°C, add 16g of trimethyl borate (0.15mol) dropwise, after dropping, raise the temperature to -10°C, then add 200ml of hydrochloric acid aqueous solution dropwise; separate the liquid, and extract the aqueous phase with 100ml×2 ethyl acetate Twice, the organic phase was combined and spin-dried to obtain a white solid; the liquid phase purity of the white solid was 99.0%, the theoretical yield was...
Embodiment 3
[0082] According to the synthesis routes provided in Examples 1 and 2, without substantial adjustments to the preparation steps, only the raw materials were replaced with corresponding groups of raw materials, and the compound with the following structure was synthesized:
[0083]
[0084]
[0085]
[0086]
[0087]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com