Liquid crystal compound containing dihydrobenzofuran and preparation method and application thereof
A compound and liquid crystal technology, applied in chemical instruments and methods, organic chemistry, liquid crystal materials, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0112] Embodiment 1, compound II Synthesis
[0113]
[0114] Step 1: Synthesis of II-a
[0115] Add 17.4g (0.1mol) 2-butyl benzofuran (reactant) P1, 2gPd / C (catalyst) 50ml toluene (solvent) 50ml ethanol (solvent) in reaction flask, hydrogenate 10 hours under 1MPa, filter The catalyst was removed, and the solvent was evaporated to dryness to obtain product 17g (II-a). Yield 96.6% GC99.5%
[0116] Synthesis of Step 2II-b
[0117] Add 17.6g (0.1mol) 2-butyl chroman (reactant) in reaction flask, methylene dichloride 100ml (solvent), add N-bromosuccinimide (reactant) 21.4g ( 0.12mol), heated to reflux for 2 hours. Add 100ml of water, separate the liquid, evaporate the solvent to dryness, and collect the 130°C / 2mmhg fraction by vacuum distillation to obtain 20g of the product (II-b). Yield 78% GC98.5%
[0118] Synthesis of Step 3II-c
[0119] Add 25.5g (0.1mol) of 2-butyl-5-bromochroman (reactant) and 80ml of tetrahydrofuran (solvent) into the reaction flask, protect it ...
Embodiment 2
[0130] Embodiment 2, compound III Synthesis
[0131]
[0132] In the reaction flask, add 24.2g (0.11mol) 3-butyl-5-boronic acid base chroman (reactant), 48.3g (0.1mol) P3 (intermediate P3 according to literature Detlef Pauluth et al J. Mater.Chem.2004, 141219-1227 synthesized) (reactant), 15g sodium carbonate, 0.3g tetrakistriphenylphosphine palladium (catalyst), 100ml toluene (solvent), 100ml water, 100ml ethanol (solvent), heated to reflux After 4 hours, 100ml of water solution was added, the toluene layer was evaporated to dryness, and the product (III) was obtained by column chromatography and recrystallization of 3 times petroleum ether, 40.5g, yield 70%, GC99.9%.
[0133] The structural verification data of this product are as follows:
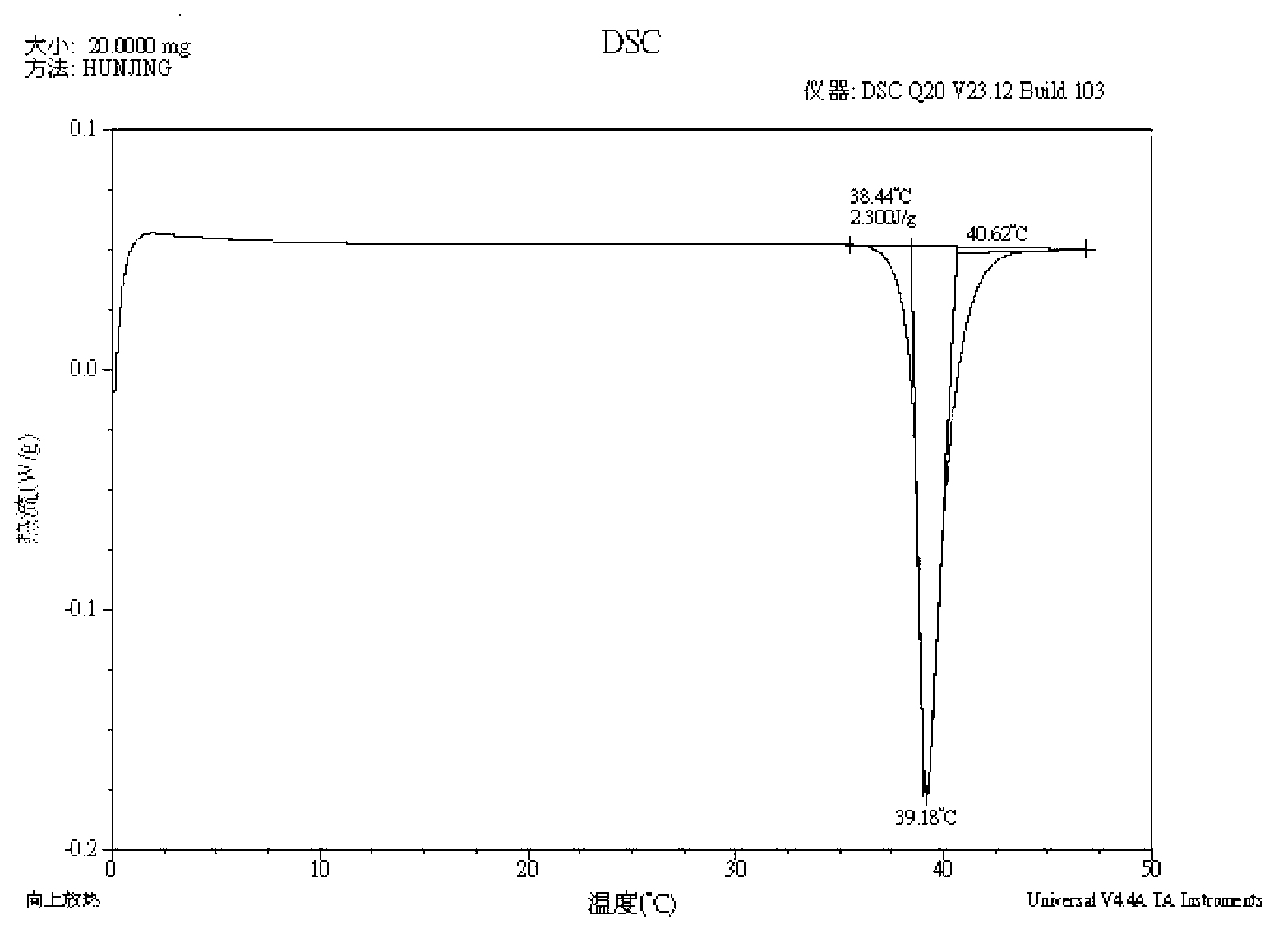
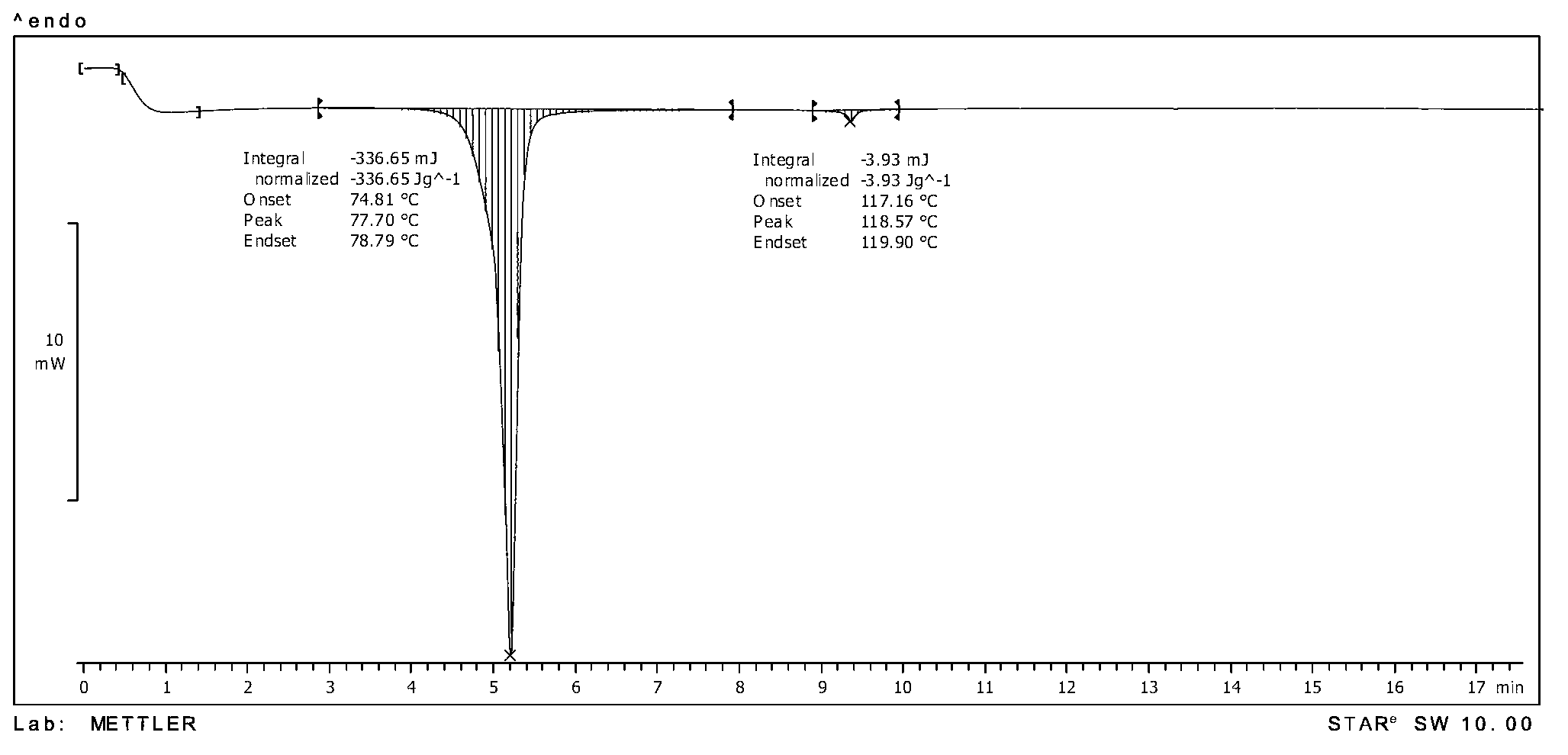
[0134] MP: 74.81℃, differential thermogram as shown image 3 shown;
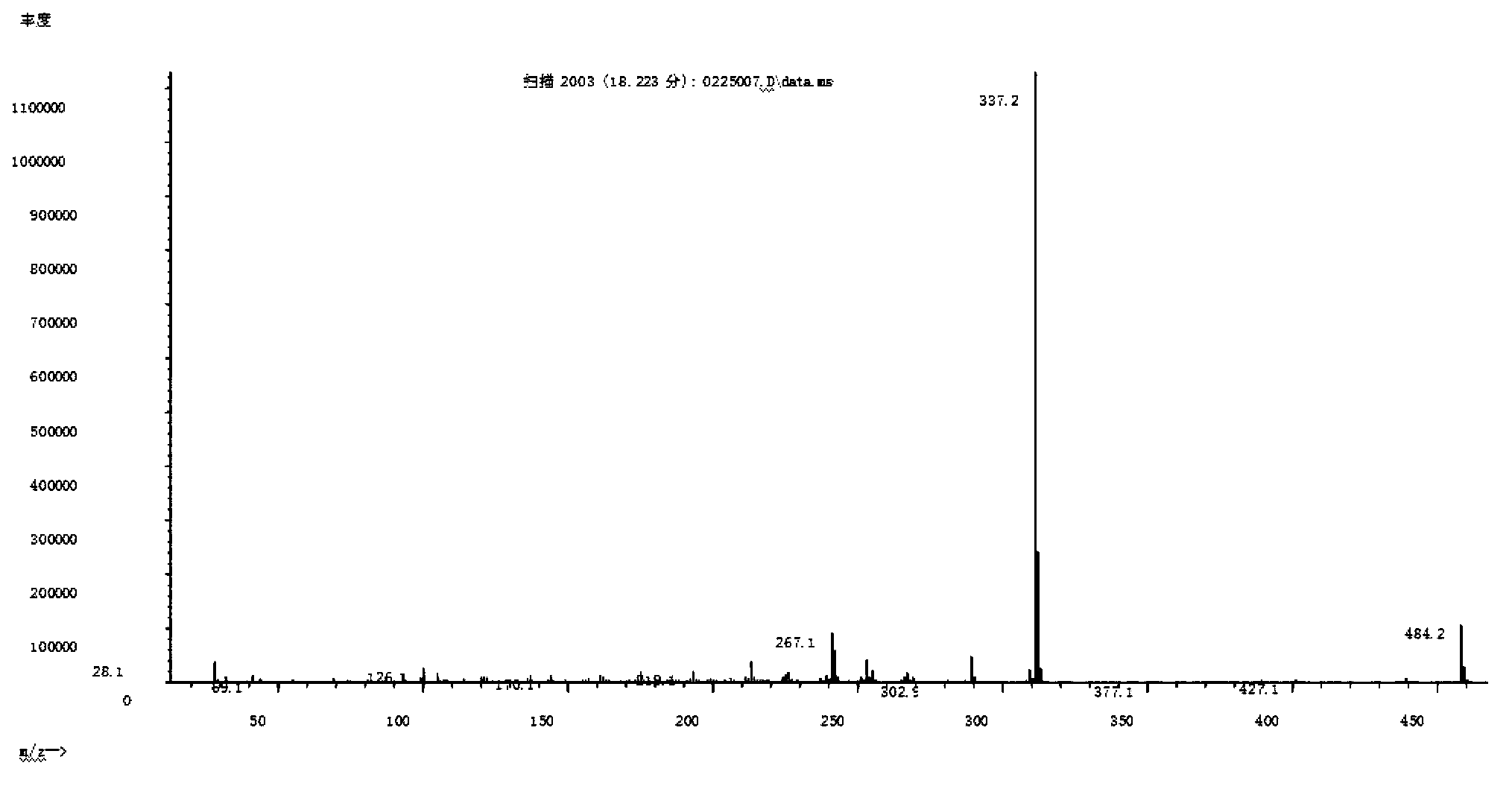
[0135] MS: m / s% 578 (30.2), 509 (2.2), 431 (100), 362 (71.9), 187 (71.8), mass spectrogram as Figure 4 shown;
[0136] 1H-NMR: δ(ppm:) 0.90(t, 3H), 1.30(m, ...
Embodiment 3~68
[0142] According to the method of the aforementioned Examples 1 and 2, only the substituents in the reactants were replaced with the substituents in the target product to obtain the compounds belonging to formula I obtained in the following Examples 3-68.
[0143]
[0144]
[0145]
[0146]
[0147]
[0148]
[0149]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com