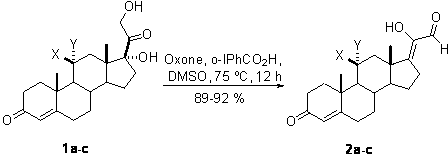
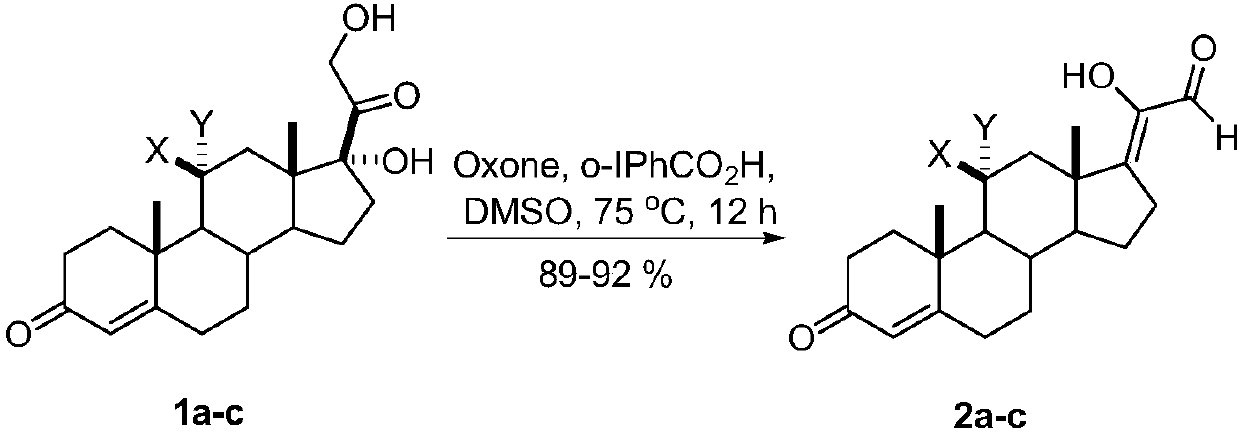
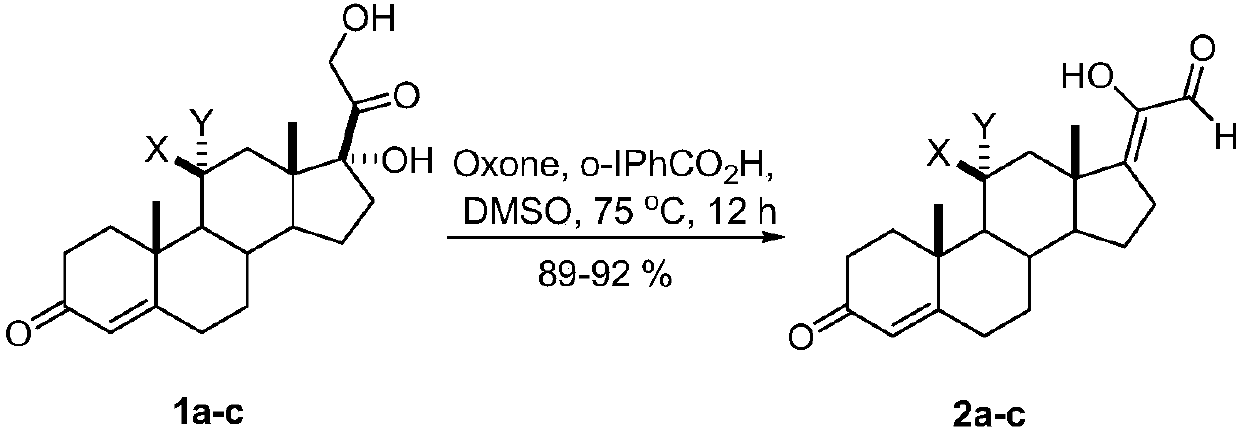
Synthetic method of 17z-(1-hydroxyl-2-oxo-1-ethylene) androstenone derivatives
A technology for the synthesis of androstenone and its synthesis method, which is applied in the field of synthesis of 17Z-androstenone, can solve the problems of difficult separation, difficult separation of isomers, and low stereoselectivity of products, and achieve high yield, highly selective effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0013] A kind of synthetic method of 17Z-(1-hydroxyl-2-oxo-1-ethylene) androstenone derivative of the present invention comprises the following steps:
[0014] A mixture of glucocorticoids is produced by mixing the catalyst o-iodobenzoic acid and the oxidant potassium persulfate, adding the solvent DMSO, reacting with mixing and stirring, quenching the reaction, and then extracting, washing, drying, and evaporating the solvent to obtain the target compound 17Z- (1-Hydroxy-2-oxo-1-ethylidene)androstenone derivatives.
[0015]
[0016] a: X=H, Y=H; b: X, Y=O; c: X=OH, Y=H
[0017] The reaction temperature in the step of the present invention is 75° C., the reaction time is 12 hours, and the yield is 89%-92%.
[0018] test instrument
[0019] Infrared spectral analysis: Bruker tensor 27 infrared spectrometer, KBr tablet method, the detection range is 4000 ~ 400cm -1 ; Melting point measurements: WRR melting point apparatus, data uncorrected. 1 H-NMR and 13 C-NMR analysis:...
Embodiment 117
[0022] Example 1 17Z-(1-hydroxy-2-oxo-1-ethylidene)androstenone (2a)
[0023] Take 1.038g (3mmol) of compound 1a, 0.93g (3.75mmol) of o-iodobenzoic acid, 2.30g (3.75mmol) of potassium hydrogen persulfate, add 3.5mL of DMSO, and stir the reaction at 75°C for 12h under nitrogen protection. The reaction mixture was quenched with 3 mL of water, extracted twice with 20 mL of chloroform each time, the combined organic phases were washed successively with 10% NaOH, water and saturated brine, Na 2 SO 4 dry. The solvent was evaporated and the residue was chromatographed (silica gel, EtOAc / DCM: 1 / 12) to give the product 17Z-(1-hydroxy-2-oxo-1-ethylidene)androstenone (2a) as a white solid 878 mg, 89% yield: mp 150-152°C (EtOAc / hexanes): 149-152°C); 1 H-NMR (CDCl 3 ,ppm):δ9.586(s,1H),5.746(s,1H),5.587(s,1H),1.218(s,3H),1.023(s,3H); 1 H-NMR (CDCl 3 +D 2 O, ppm): δ9.583 (s, 1H), 5.748 (s, 1H), 1.219 (s, 3H), 1.023 (s, 3H); 13 C-NMR (CDCl 3 ,ppm): δ199.52,186.09,170.87,147.02,141.83...
Embodiment 217
[0024] Example 2 17Z-(1-hydroxy-2-oxo-1-ethylene)-4-androst-3,11-enedione (2b)
[0025]Take 1.083g (3mmol) of compound 1a, 0.93g (3.75mmol) of o-iodobenzoic acid, 2.30g (3.75mmol) of potassium hydrogen persulfate, add DMSO 3.5mL, and stir at 75°C for 12h under nitrogen protection. The reaction mixture was quenched with 3 mL of water, extracted twice with 20 mL of chloroform each time, the combined organic phases were washed successively with 10% NaOH, water and saturated brine, Na 2 SO 4 dry. The solvent was evaporated and the residue was chromatographed (silica gel, EtOAc / DCM: 1 / 12) to give the product 17Z-(1-hydroxy-2-oxo-1-ethylidene)-4-androst-3 as a white solid , 11-enedione (2b) 0.946mg, yield 92%: mp190-192°C (EtOAc / Hexanes): 188-192°C); 1 H-NMR (CDCl 3 ,ppm): δ9.595(d,J=0.9Hz,1H),5.740(s,1H),5.630(d,J=0.9Hz,1H),3.250(d,J=13.2Hz,1H),1.432 (s,3H),0.978(s,3H); 1 H-NMR (CDCl 3 +D 2 0, ppm): δ9.592(s, 1H), 5.746(s, 1H), 3.248(d, J=13.2Hz, 1H), 1.432(s, 3H), 0.979(s,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com