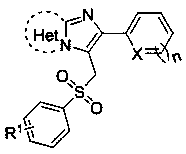
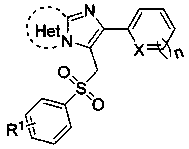
3-(Benzenesulfonylmethyl)imidazoheterocyclic compounds and synthetic method thereof
A technology of benzenesulfonylmethyl and benzenesulfonylmethyl isonitrile, which is applied in the field of organic compound synthesis and application, can solve problems such as unreported sulfonyl methylation reaction, and achieve the effect of enriching functional group reaction types
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] Compound 2-(furan-2-yl)-3-(tosylmethyl)imidazo[1,2- a ] The structure of pyridine is:
[0025] ;
[0026] Preparation method: Add 0.1 mmol of 2-furylimidazo[1,2-a]pyridine compound, 0.2 mmol of p-toluenesulfonylmethyl isocyanide, 0.02 Mmol of ferric chloride, 2 mL of water, react at 100°C for 36 hours; after the reaction, extract with dichloromethane, concentrate under reduced pressure and then chromatographically separate (silica gel 200-300 mesh), eluent: ethyl acetate / petroleum Gradient elution with ether, the ratio is from 0 / 100 to 100 / 0), and drying to obtain a white solid with a yield of 90%. m.p. = 163-164°C. 1 H NMR (600 MHz, CDCl 3 ) δ 8.40 (d, J = 6.9 Hz, 1H), 7.60(d, J = 9.1 Hz, 1H), 7.38 (d, J = 8.2 Hz, 2H), 7.32-7.29 (m, 1H), 7.13 (dd , J= 1.6, 0.5 Hz, 1H), 7.06 (d, J = 7.9 Hz, 2H), 6.94 (td, J = 6.8, 1.0 Hz, 1H), 6.66 (d, J = 1.1 Hz, 1H), 6.31 (dd, J = 3.4, 1.8 Hz, 1H), 5.08 (s, 2H), 2.27(s, 3H). 13 C NMR (150 MHz, CDCl 3 ) δ 149.1, 146.3, 145.1,...
Embodiment 2
[0028] Compound 2-(thiophen-2-yl)-3-(tosylmethyl)imidazo[1,2- a ] The structure of pyridine is:
[0029] ;
[0030] Preparation method: Add 0.1 mmol of 2-thienylimidazo[1,2-a]pyridine compound, 0.3 mmol of p-toluenesulfonylmethyl isocyanide, 0.03 Mmol of ferric chloride, polyethylene glycol 400 2mL, react at 100°C for 18 hours; after the reaction, extract with dichloromethane, concentrate under reduced pressure and then chromatographically separate (silica gel 200-300 mesh), eluent: ethyl acetate Gradient elution with ester / petroleum ether, the ratio is from 0 / 100 to 100 / 0), and dried to obtain a white solid with a yield of 87%. m.p. = 161-162°C. 1 H NMR (600 MHz, CDCl 3 ) δ 8.33 (d, J = 6.9 Hz, 1H),7.64 (d, J = 9.1 Hz, 1H), 7.50 (d, J = 8.3 Hz, 2H), 7.31-7.26 (m, 2H), 7.15(d , J = 8.0 Hz, 2H), 7.02 (dd, J = 3.6, 1.0 Hz, 1H), 6.95 (dd, J = 5.0, 3.7Hz, 1H), 6.90 (td, J = 6.8, 1.0 Hz, 1H) , 4.90 (s, 2H), 2.35 (s, 3H). 13 C NMR (150 MHz, CDCl 3 ) δ 146.0, 145.5, 141.7, ...
Embodiment 3
[0032] The compound 6-phenyl-5-(tosylmethyl)imidazo[2,1- b ] The structural formula of thiazole is:
[0033] ;
[0034] Preparation method: Add 0.1 mmol of 6-phenylimidazo[2,1- b ] Thiazole compound, 0.3mmol of p-toluenesulfonylmethyl isonitrile, 0.01mmol of iron trichloride, water and polyethylene glycol 400 (volume ratio 7:3) 2 mL, 120 ℃ of reaction 24 hours; , extracted with dichloromethane, concentrated under reduced pressure and then chromatographically separated (silica gel 200-300 mesh), eluent: ethyl acetate / petroleum ether gradient elution, ratio from 0 / 100 to 100 / 0), and dried to obtain a white solid , 85% yield. m.p. = 39-40℃. 1 H NMR (600 MHz, CDCl 3 ) δ 7.71 (d, J = 4.5Hz, 1H), 7.45 (d, J = 8.2 Hz, 2H), 7.24-7.23 (m, 3H), 7.18-7.14 (m, 4H), 6.90(d, J = 4.5 Hz, 1H), 4.69 (s, 2H), 2.37 (s, 3H). 13 C NMR (150 MHz, CDCl 3 ) δ150.9, 148.7, 145.4, 134.1, 133.2, 129.9, 128.29, 128.27, 127.8, 127.6, 119.1, 112.6, 109.8, 53.6, 21.6. HRMS (positive ESI): [M+H] + ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com