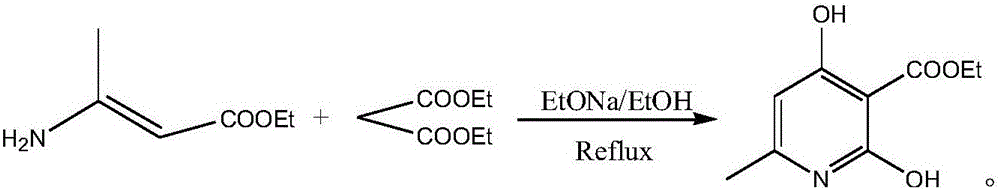Method for preparing 2,4-dyhydroxy-6-methylnicotinate
A technology of ethyl methylnicotinate and diethylmalonate, applied in the field of preparation of ethyl 2,4-dihydroxy-6-methylnicotinate, can solve unfavorable industrial production, low yield, production High cost and other issues, to achieve the effect of green reaction system, simple preparation method, and less harsh reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0014] A preparation method of ethyl 2,4-dihydroxy-6-methylnicotinate, ethyl 3-aminocrotonate and diethyl malonate successively undergo Claisen ester condensation and condensation under the action of sodium ethoxide Dickermann ester condensation reaction, after the reaction is over, cool the reaction system to 50°C, concentrate under reduced pressure, add basic activated carbon to the concentrated solution, filter, cool the filtrate to below 0°C, adjust the pH of the filtrate with ammonium chloride value to 2, the resulting solid was dried at a temperature of 50°C to obtain ethyl 2,4-dihydroxy-6-methylnicotinate. The temperature of the reaction is 80°C, the reaction time is 20h, the system solvent is absolute ethanol, and the weight ratio of ethyl 3-aminocrotonate, diethyl malonate and sodium ethylate is 0.8:1.5:1 .
Embodiment 2
[0016] A preparation method of ethyl 2,4-dihydroxy-6-methylnicotinate, ethyl 3-aminocrotonate and diethyl malonate successively undergo Claisen ester condensation and condensation under the action of sodium ethoxide Dickermann ester condensation reaction, after the reaction is over, cool the reaction system to 60°C, concentrate under reduced pressure, add basic activated carbon to the concentrated solution, filter, cool the filtrate to below 0°C, adjust the pH of the filtrate with ammonium chloride value to 3, the resulting solid was dried at a temperature of 60° C. to obtain ethyl 2,4-dihydroxy-6-methylnicotinate. The temperature of the reaction is 90°C, the reaction time is 30h, the system solvent is absolute ethanol, and the weight ratio of ethyl 3-aminocrotonate, diethyl malonate and sodium ethylate is 1.5:2.5:1 .
Embodiment 3
[0018] A preparation method of ethyl 2,4-dihydroxy-6-methylnicotinate, ethyl 3-aminocrotonate and diethyl malonate successively undergo Claisen ester condensation and condensation under the action of sodium ethoxide Dickermann ester condensation reaction, after the reaction is over, cool the reaction system to 53°C, concentrate under reduced pressure, add basic activated carbon to the concentrated solution, filter, cool the filtrate to below 0°C, adjust the pH of the filtrate with ammonium chloride value to 2.4, the resulting solid was dried at a temperature of 54°C to obtain ethyl 2,4-dihydroxy-6-methylnicotinate. The temperature of the reaction is 83°C, the reaction time is 24h, the system solvent is absolute ethanol, and the weight ratio of ethyl 3-aminocrotonate, diethyl malonate and sodium ethylate is 1:1.8:1 .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com