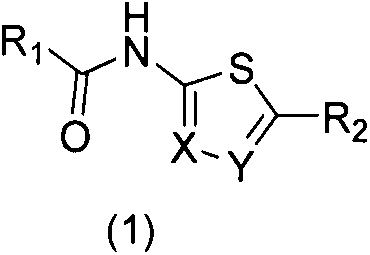
Medical application of n-(thiophene-2)amide derivatives in pyrazinamide-resistant tuberculosis and tuberculosis treatment
A technology resistant to pyrazinamide and amide compounds, applied in the field of medicine, can solve the problems of inability to interact, inability of POA to combine with RpsA, inability to inhibit trans-translation process, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0013] The present invention mainly relates to the discovery process of compounds containing N-(thiophene-2) amides, and the research on the interaction between compounds and proteins at the molecular level (fluorescence quenching titration method), and the in vitro anti-mycobacterium tuberculosis and resistance to PZA at the cellular level Antibacterial test of Mycobacterium tuberculosis.
[0014] Drugs and Reagents
[0015] sample
[0016]
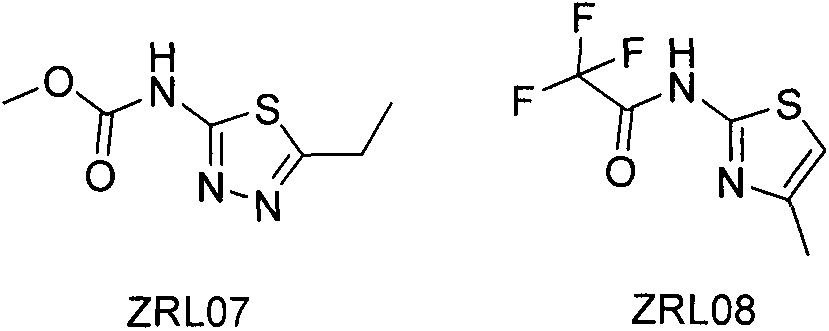
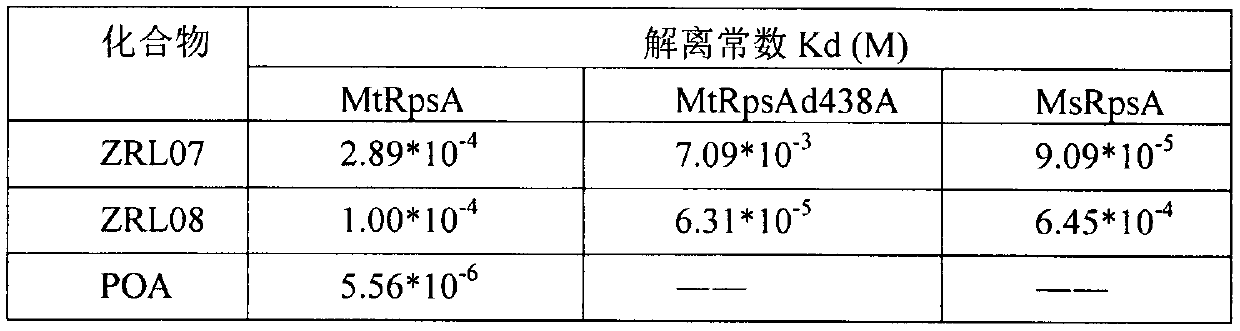
[0017] Reagents: control POA and PZA and compounds to be determined ZRL07 and 08; expressed and purified protein samples: MtRpsA (285-476), MtRpsAd438A (285-476) and MsRpsA (285-474); Mycobacterium tuberculosis strain: wild type All-sensitive strains (H37Ra) and 3 clinically found PZA-resistant strains (PZA069, PZA080 and PZA073); other conventional chemical reagents: DMSO, D 2 O, phosphate buffer saline (PBS), etc.
[0018] Instruments: Fluorescence spectrophotometer (F-7000, Hitachi, Japan), Roche culture medium, constant tempera...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com