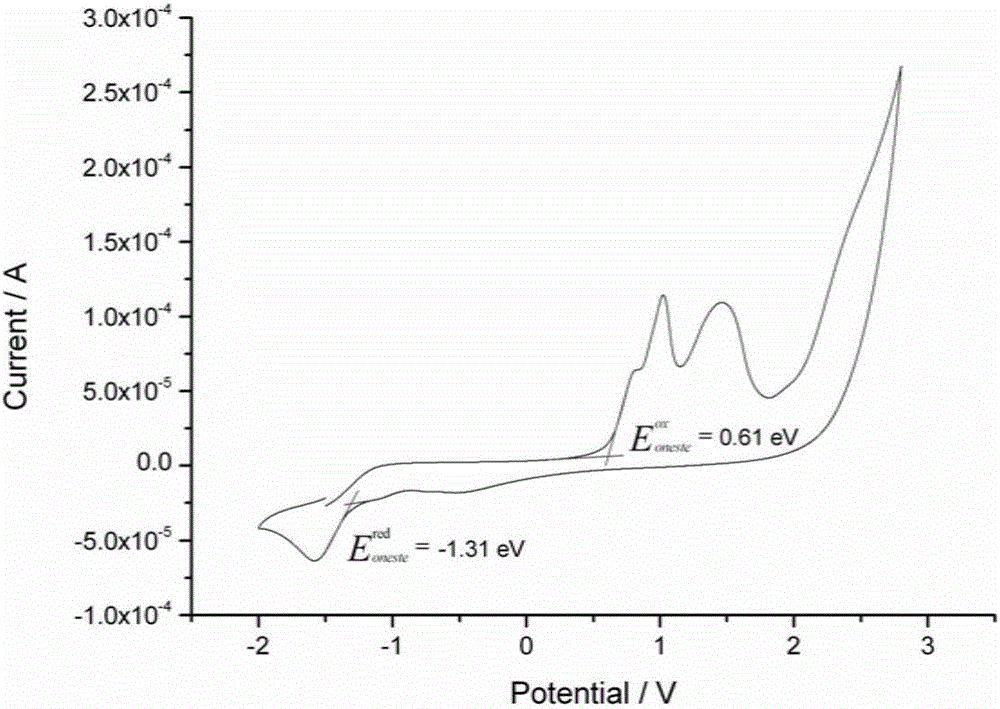
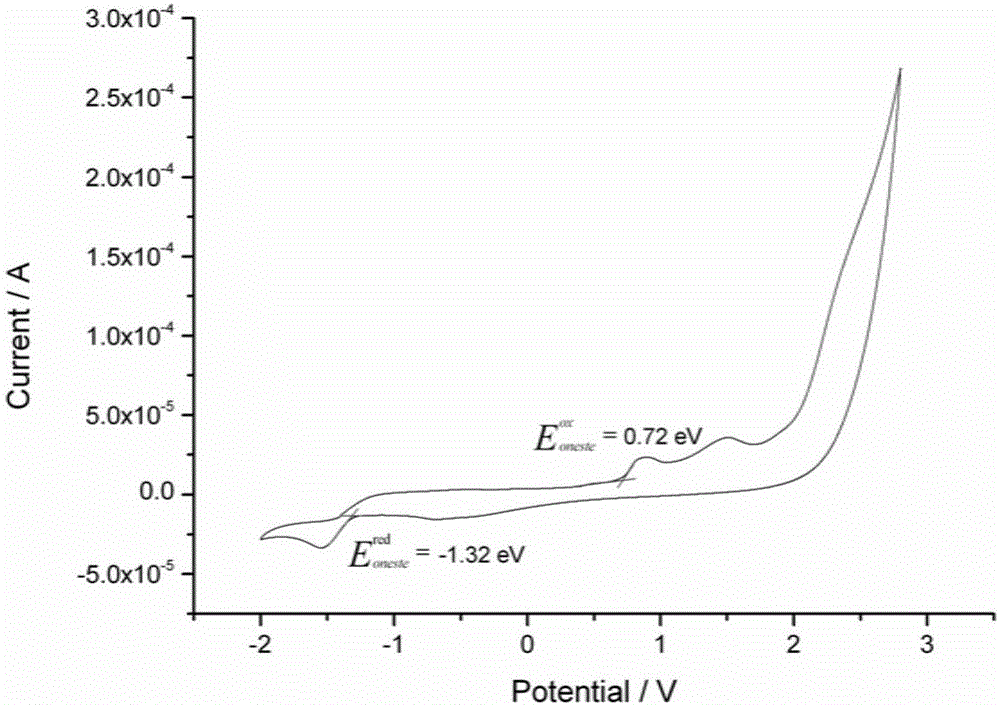
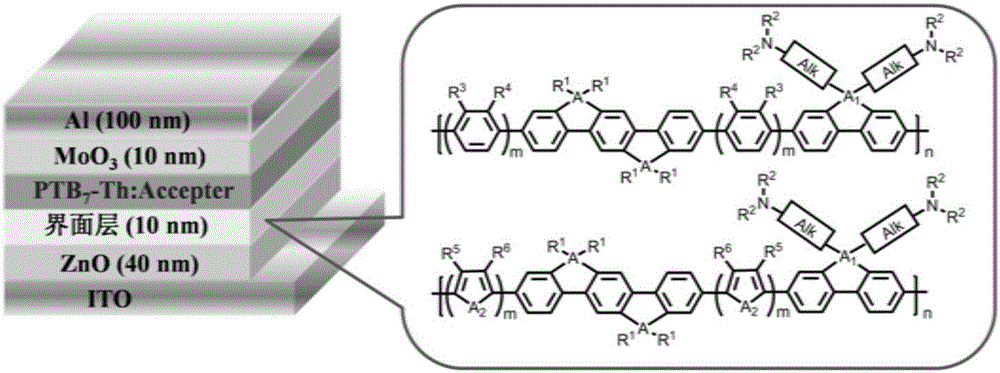
Indenofluorene derivative based solar cell interface material
A technology of solar cells and interface materials, applied in the field of solar cell interface materials and their preparation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0097] Embodiment 1: the synthesis of liposoluble unit
[0098]
[0099] (1) Preparation of 6,6,12,12-tetra-n-octylinden[1,2-b]fluorene (5)
[0100] 2.54g of compound 4 (10mmol) was added in 100mL of tetrahydrofuran / diethyl ether / methyl tert-butyl ether in one or more solvents, under Ar protection, n-BuLi (2.5M, 30mmol, 12mL) was added dropwise at -20°C and After slowly rising to room temperature, stir for 1.5 h, then cool to -20° C., add n-octyl bromide (30 mmol), then warm to room temperature and stir for 4 h. After adding 30mmol n-BuLi and 30mmol n-octyl bromide again, react overnight at room temperature and spot the plate to detect the reaction. After extraction, the solvent was spin-dried and petroleum ether was added to precipitate a solid. Column chromatography collected 8.7 g of a white solid, with a yield of 95%. 1 H NMR (500MHz, CDCl 3 ),δ(ppm):7.78(dd,J=2.0,7.0Hz,2H),7.56(s,2H),7.37(dd,J=3.0,13.5Hz,4H),7.34-7.27(m,2H) ,2.08-2.04(m,8H),1.21-1.08(m,40H),0.83(t,...
Embodiment 2
[0116] Embodiment 2: the synthesis of interface affinity unit
[0117]
[0118] (1) Preparation of 2,7-dibromo-9,9-bis(6-dimethylaminohexyl)fluorene (12)
[0119] 1,6-Dibromohexane (40mL, 256mmol), 40mL of KOH (50%) solution and tetrabutylammonium bromide (TBAB, 1.436g, 4.3mmol) were added to the reaction flask in sequence, and the temperature was raised to 75°C and added Compound 11 (5g, 15.4mmol) continued to react for 15min, cooled to room temperature and extracted with chloroform (30mLx3), washed the organic phase with 1M HCl, washed with saturated brine, dried over anhydrous magnesium sulfate, removed DCM and distilled off 1,6 under reduced pressure -Dibromohexane, the crude product was purified by silica gel column (PE).
[0120] Dissolve the above-mentioned product in 50 mL of tetrahydrofuran / diethyl ether / methyl tert-butyl ether in a certain solvent or a mixture of several solvents, add 20 equivalents of dimethylamine aqueous solution and react in the dark for 24 h...
Embodiment 3
[0123] Embodiment 3: Synthesis of silicon-containing fat-soluble unit
[0124]
[0125] (1) Preparation of 6,6,12,12-tetra-n-octyl-6,12-disilazane[1,2-b]fluorene (5)
[0126] 2.86g of compound 14 (10mmol) was added in 100mL of tetrahydrofuran / diethyl ether / methyl tert-butyl ether in one or more solvents, under Ar protection, n-BuLi (2.5M, 30mmol, 12mL) was added dropwise at -20°C and After slowly rising to room temperature, stir for 1.5 h, then cool to -20° C., add n-octyl bromide (30 mmol), then warm to room temperature and stir for 4 h. After adding 30mmol n-BuLi and 30mmol n-octyl bromide again, react overnight at room temperature and spot the plate to detect the reaction. After extraction, the solvent was spin-dried and petroleum ether was added to precipitate a solid. Column chromatography yielded 6.5 g of a white solid, Yield=88%. 1 H NMR (500MHz, CDCl 3 ), δ(ppm): 7.77(dd,J=2.0,7.0Hz,2H),7.54(s,2H),7.33(dd,J=3.0,13.5Hz,4H),7.31-7.22(m,2H) ,1.75-1.68(m,8H),1.19-1....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com