Colorectal cancer peritoneal metastasis model and establishment method thereof
A metastasis model, colorectal cancer technology, applied in botany equipment and methods, biochemical equipment and methods, pharmaceutical formulations, etc., can solve the problems of tumor inoculation failure, many complications, and long preparation cycle
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] Example 1 Establishment of peritoneal metastasis model by intraperitoneal injection of colorectal cancer cell lines
[0039] 1. Animal model
[0040] (1) intraperitoneal injection, 2*10 5 cells / only
[0041] 2. In vivo fluorescence imaging
[0042] Establishment of peritoneal metastasis model by intraperitoneal injection, and detection of tumor formation and progression by intravital fluorescence imaging.
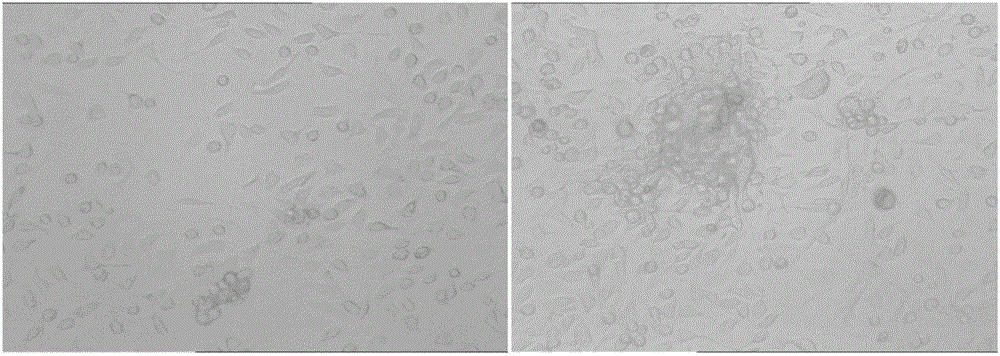
[0043] The result is as figure 1 As shown, as the modeling time prolongs, a certain number of tumor cells that can be detected by the live imager are formed in the abdominal cavity of the mouse.
[0044] 3. Laparotomy imaging
[0045] Intraperitoneal injection of 10*5 cells / only for modeling. After 21 days of modeling, the mice were sacrificed, and the mesentery was imaged.
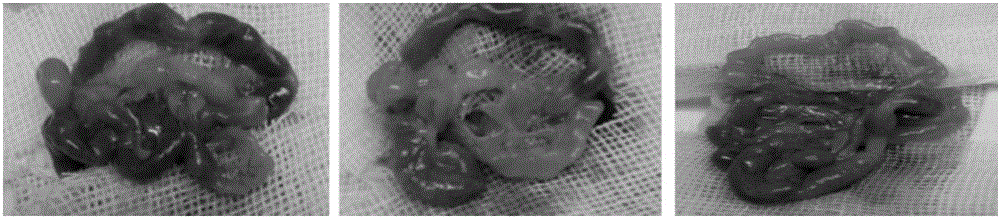
[0046] The result is as figure 2 shown. It can be seen that the mesenteric nodules of the mice are obvious, and obvious ascites is seen. The above results show that: 22 days after the ...
Embodiment 2
[0055] Example 2 Establishment of peritoneal metastasis model by in situ inoculation of cecum wall
[0056] 1. Animal model
[0057] In situ inoculation of cecum wall: 2*10 5 Cells per Matrigel were fixed on the serosa layer of the cecal wall.
[0058] 2. In vivo fluorescence imaging
[0059] Establishment of a peritoneal metastasis model inoculated with the cecal wall and detection of tumor formation and progression using intravital fluorescence imaging.
[0060] The result is as Figure 6 As shown, the results showed that a series of visceral metastases such as liver metastases and lung metastases appeared in mice 17-30 days after injection.
[0061] 3. Laparotomy imaging
[0062] The cecum wall was inoculated with 10*5 cells / only for modeling. After 21 days of modeling, the mice were sacrificed, and the mesentery was imaged.
[0063] The result is as Figure 7 As shown, it can be seen that the peritoneal wall and mesentery metastasis nodules in mice are obvious, and...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com