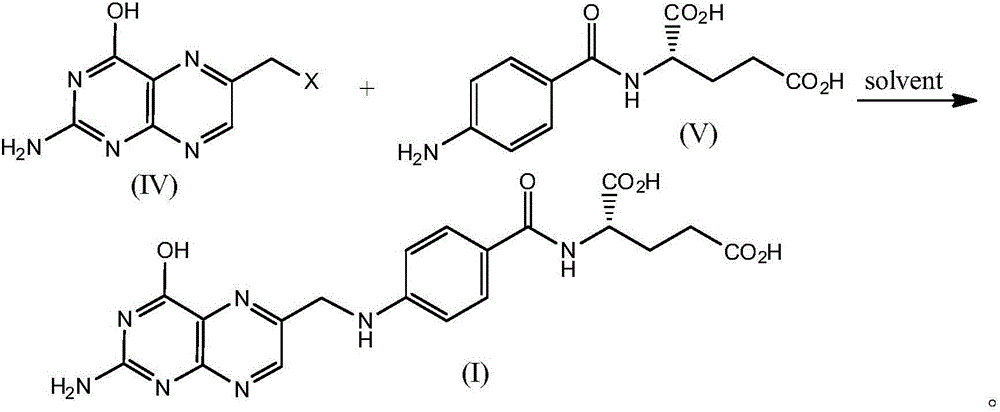
Folic acid synthesis method
A kind of synthetic method, the technology of folic acid
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
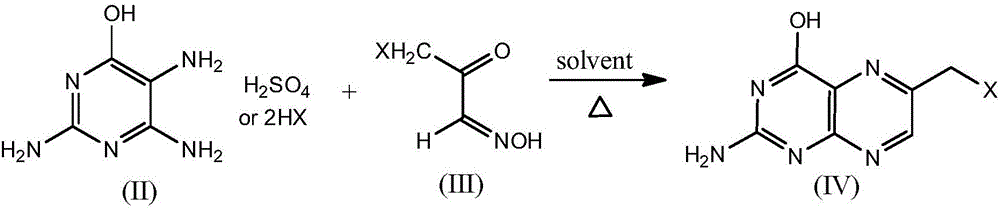
[0025] Embodiment 1: Synthesis of 2-amino-4-hydroxyl-6-bromomethylpteridine
[0026] Add 15.15 grams (50 mmol) of 2,4,5-triamino-6-hydroxypyrimidine dihydrogen bromide and 500 milliliters of methanol in the reaction flask, and add 12.45 grams (75 mmol) of 3-bromoacetoglyoxal to the suspension formed under stirring 100 ml of methanol solution of oxime, heated to reflux for 2.5 hours, rotary evaporation to remove about 480 ml of methanol, cooled to room temperature, stirring the residue with concentrated ammonia, and suction filtration to obtain 2-amino-4-hydroxy-6-bromomethyl The crude pteridine product was washed with methanol and dried in a vacuum oven heated to 60° C. The yield was 12.2 g, and the yield was 95%.
Embodiment 2
[0027] Embodiment 2: Synthesis of 2-amino-4-hydroxyl-6-bromomethylpteridine
[0028] Add 15.15 grams (50 mmol) of 2,4,5-triamino-6-hydroxypyrimidine dihydrogen bromide and 500 ml of 95% ethanol to the reaction flask, and add 8.30 grams (50 mmol) of 3-bromo 75 ml of 95% ethanol solution of acetone aldoxime, heated and refluxed for 3 hours, removed about 450 ml of 95% ethanol by rotary evaporation, cooled to room temperature, and the residue was neutralized with 7% sodium carbonate aqueous solution under stirring, and suction filtered to obtain 2-amino - The crude product of 4-hydroxy-6-bromomethylpteridine was washed with 95% ethanol, and dried in a vacuum oven heated to 70° C. The yield was 11.14 g, and the yield was 87%.
Embodiment 3
[0029] Example 3: Synthesis of 2-amino-4-hydroxyl-6-chloromethylpteridine
[0030] Add 10.70 grams (50 mmol) of 2,4,5-triamino-6-hydroxypyrimidine dihydrochloride and 450 milliliters of methanol to the reaction flask, and add 9.11 grams (75 mmol) of 3-chloroacetone aldoxime to the suspension formed under stirring 90 ml of methanol solution, heated to reflux for 3 hours, rotary evaporated to remove about 420 ml of methanol, cooled to room temperature, stirred, the residue was neutralized with concentrated ammonia water, and suction filtered to obtain 2-amino-4-hydroxyl-6-chloromethyl The crude product of pteridine was washed with methanol and dried in a vacuum oven heated to 60° C. The yield was 9.42 g, and the yield was 89%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com