New anti-human pai-1 antibody
A PAI-1, antibody technology, applied in the direction of antibodies, antibody medical components, recombinant DNA technology, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0184] (Example 1: Study on immune antigen and preparation of hybridoma producing anti-human PAI-1 antibody)
[0185]In order to obtain highly selective antibodies against active human PAI-1, optimal human PAI-1 peptide antigens were studied. As a result of studying the length and amino acid sequence of the peptide, it was found that Multiple Antigen Peptide 8 (Multiple Antigen Peptide 8, MAP8) (J. Biol. Chem., 1988, Vol. 263, No. 4, p. 1719-1725) peptide immunization can obtain antibodies that neutralize active human PAI-1 in plasma. The results of further original research on the protein linked to the antigen, the immunization method, etc. showed that the peptide with the keyhole limpet hemocyanin (Keyhole Limpet Hemocyanin, KLH) protein linked to the C-terminus of the peptide antigen and the recombinant active human When PAI-1 (Molecular Innovation, PAI-A) is mixed and immunized at the same time, the active form that strongly neutralizes the active form of human PAI-1 in p...
Embodiment 2
[0187] (Example 2: ELISA detection)
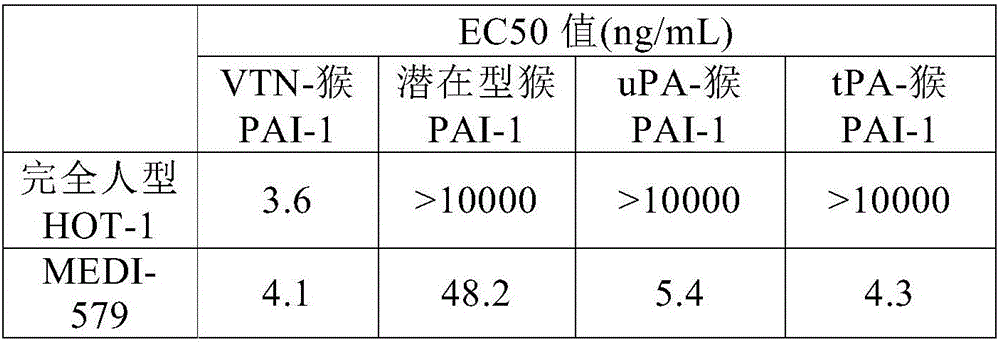
[0188] To measure the antigen-specific binding activity of antibodies, ELISA assays were used. Here, in order to evaluate the selectivity to active human PAI-1, a plate prepared by immobilizing vitronectin and adding active human PAI-1 was used, and latent human PAI-1 was added instead of active human PAI. -1 and made the board to test.
[0189] Dilute vitronectin (BD Biosciences, 354238) with phosphate-buffered saline (PBS) to 1000 ng / mL, add 100 μL per well to a Nunc Maxisorp transparent 96 plate (Nunc company), and place the plate at 4°C Overnight, thereby immobilizing vitronectin. The vitronectin solid phase was removed by reverse centrifugation, and recombinant active human PAI-1 (Molecular Innovation, PAI-A) or recombinant latent human PAI diluted to a concentration of 1000 ng / mL with PBS was added at 100 μL per well. -1 (Molecular Innovation, PAI-L), left still at 4°C for 1 hour. The solution was removed by reverse centrifugatio...
Embodiment 3
[0192] (Example 3: Production of fully human antibody)
[0193] The above antibodies are antibodies in which the variable regions are derived from humans and the constant regions are derived from mice. Therefore, the present inventors constructed an expression vector containing both heavy chain and light chain genes using a GS vector (Lonza Corporation), which is a vector for expression in mammalian cells, and produced a fully human antibody. Specifically, for HOT-1 identified in Example 2, RNA was extracted from the hybridoma, and cDNA was prepared using a cDNA amplification kit (SMARTer RACE cDNA amplification kit; Clontech). Next, the variable regions of the heavy and light chains are extended and amplified using the polymerase chain reaction (PCR). The gene encoding the signal sequence (Nigel Whittle et al., Protein Engineering, 1987, Vol.1, No.6, p.499-505) was connected to the 5' side of the heavy chain variable region gene of HOT-1, and then at the 3' The constant reg...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com