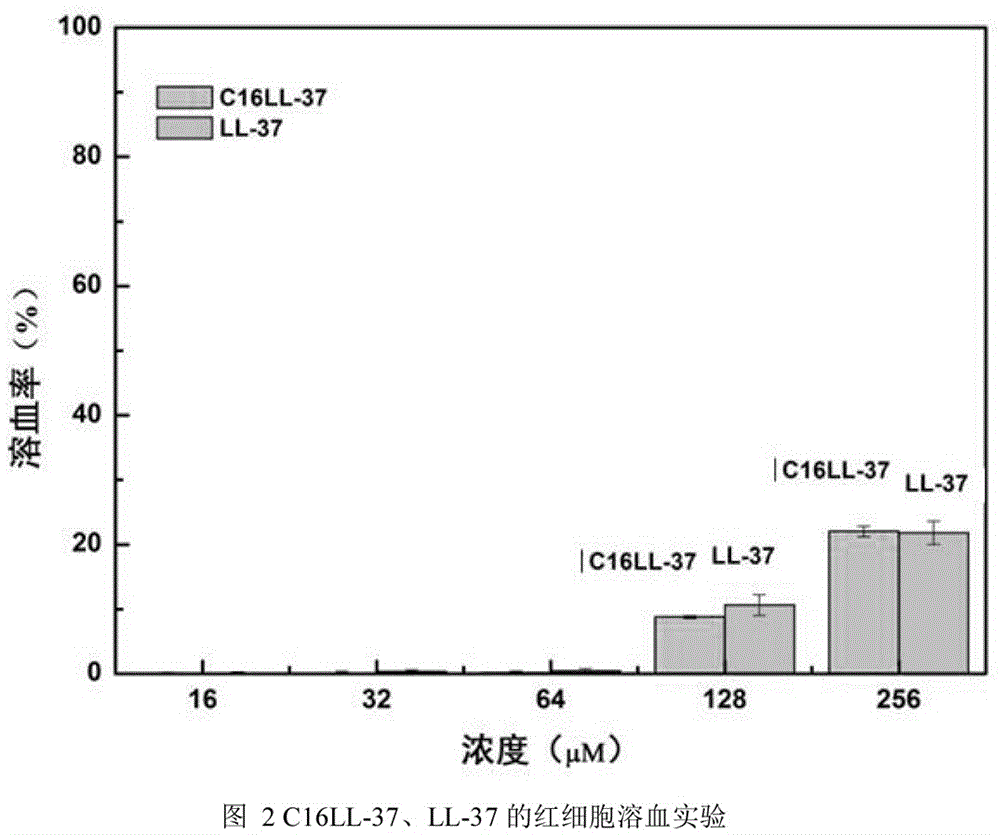
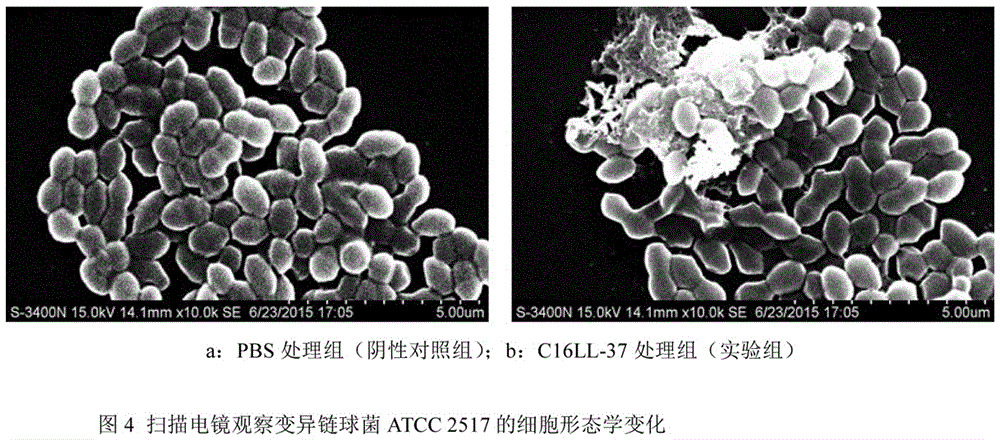
Recombinant human-derived antimicrobial peptide C16LL-37 and application method thereof in streptococcus mutans bioactivity role
A technology of C16LL-37, Streptococcus mutans, applied in the field of bioengineering
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0075] A recombinant human antimicrobial peptide C16LL-37, synthesized by standard solid-phase synthesis technology, the amino acid sequence of human antimicrobial peptide LL-37 is LLGDFRKSKEKIGKEFKRIVQRIKDFLRNLVPRTES and the C-terminal 16 amino acid sequence CSPC16 of Streptococcus mutans CSP is TFFRLFNRSFTQALGK through Linker-GGG-connection, synthetic specific targeting ability of recombinant human-derived antimicrobial peptide C16LL-37 as
[0076] TFFRLFNRSFTQALGK-GGG-LLGDFFRKSKEKIGKEFKRIVQRIKDFLRNLVPRTES-NH 2 .
[0077] A preparation method of recombinant human-derived antimicrobial peptide C16LL-37, comprising the steps of:
[0078] A. Resin swelling
[0079] Before use, soak the reaction tube in dichloromethane DCM with a concentration of 7.6ml / g for 5 hours, then weigh 5g of Fomc-Rink-amide-MBHA resin and add it to the reaction tube, and add a concentration of 7.6ml / g along the tube wall 50ml of dichloromethane DCM to make it immerse the resin, after soaking for 2-3h...
PUM
| Property | Measurement | Unit |
|---|---|---|
| purity | aaaaa | aaaaa |
| turbidity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com