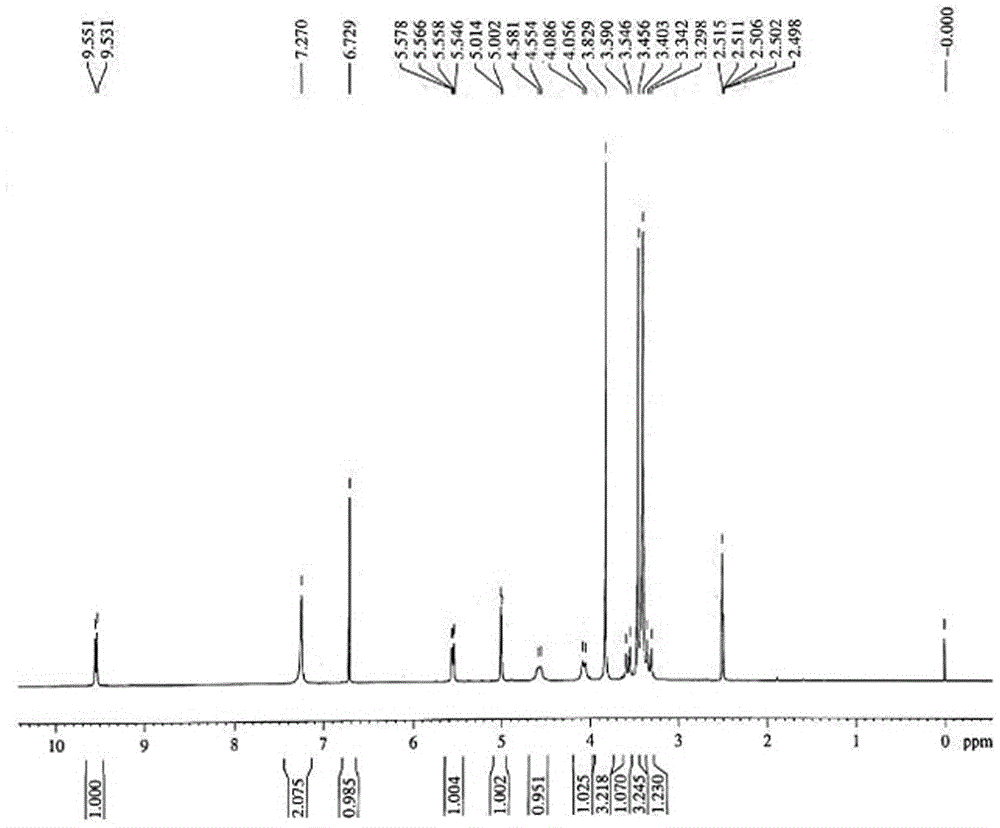
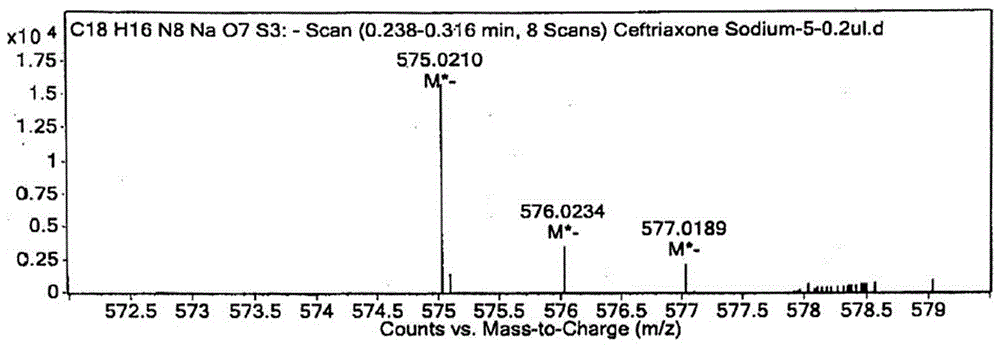
Preparation method and product of ceftriaxone sodium sterile powder
A technology of ceftriaxone sodium and sodium bisulfite, which is applied in the field of medicine, can solve problems such as unfavorable washing and separation, poor crystal form and poor quality of ceftriaxone sodium, achieve shortened production cycle, easy control of reaction conditions, and improve effectiveness Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] A preparation method of ceftriaxone sodium aseptic powder, comprising the following steps:
[0032] (a) In a clean glass kettle, first cool 12 L of dichloromethane and 1.1 L of ethanol with a mass concentration of 90% to 0-5 °C, then add 0.05 kg of sodium bisulfite, 1.1 kg of AE-activated The ester and 1 kg 7-ACT were dissolved to obtain a mixed solution;
[0033] (b) Add 0.6 kg of triethylamine dropwise to the mixture in step (a) for reaction within 1 hour while maintaining the pH of the system at 8.0 to 8.5 and the temperature at 0 to 5°C. Use HPLC to detect the residual amount of 7-ACT <0.1 %, the reaction is complete;
[0034] (c) After the reaction is finished, add 4.5 kg of sodium bisulfite aqueous solution with a mass concentration of 0.1% for extraction. After the water phase is filtered under reduced pressure, add 5.6 kg of sodium isooctanoate and 2.8 kg sodium acetate reacts into a salt;
[0035] (d) After the salt-forming reaction is complete, add activate...
Embodiment 2
[0037] A preparation method of ceftriaxone sodium aseptic powder, comprising the following steps:
[0038] (a) In a clean glass kettle, first cool 10 L of dichloromethane and 1.2 L of ethanol with a mass concentration of 90% to 0–5°C, then add 0.04 kg of sodium bisulfite, 1.1 kg of AE-activated The ester and 1 kg 7-ACT were dissolved to obtain a mixed solution;
[0039] (b) Add 0.6 kg of triethylamine dropwise to the mixture in step (a) within 1 hour for reaction while maintaining the pH of the system between 8.0 and 8.5 and the temperature at 0 to 5°C. Use HPLC to detect the residual amount of 7-ACT When <0.1%, the reaction is complete;
[0040] (c) After the reaction is over, add 4.2kg of sodium bisulfite aqueous solution with a mass concentration of 0.1% for extraction. After the water phase is filtered under reduced pressure, add 5.7kg of sodium isooctanoate and 2.8 kg sodium acetate reacts into a salt;
[0041] (d) After the salt-forming reaction is complete, add activ...
Embodiment 3
[0043] A preparation method of ceftriaxone sodium aseptic powder, comprising the following steps:
[0044] (a) In a clean glass kettle, first cool 12 L of dichloromethane and 1.2 L of ethanol with a mass concentration of 90% to 0-5°C, then add 0.05 kg of sodium bisulfite, 1.05 kg of AE-active The ester and 1 kg 7-ACT were dissolved to obtain a mixed solution;
[0045] (b) Add 0.65 kg of triethylamine dropwise to the mixture in step (a) for reaction within 1 hour while maintaining the pH of the system between 8.0 and 8.5 and the temperature at 0 to 5°C. Use HPLC to detect the residual amount of 7-ACT When <0.1%, the reaction is complete;
[0046](c) After the reaction is over, add 4.4kg of sodium bisulfite aqueous solution with a mass concentration of 0.1% for extraction. After the water phase is filtered under reduced pressure, add 5.5kg of sodium isooctanoate and 2.8 kg sodium acetate reacts into a salt;
[0047] (d) After the salt-forming reaction is complete, add activat...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com