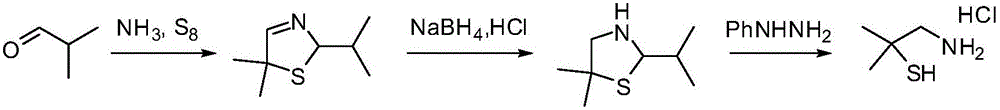
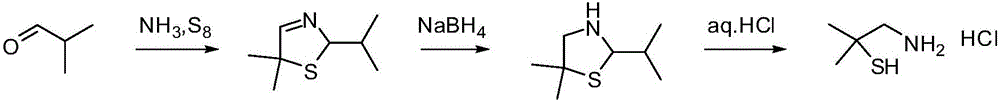
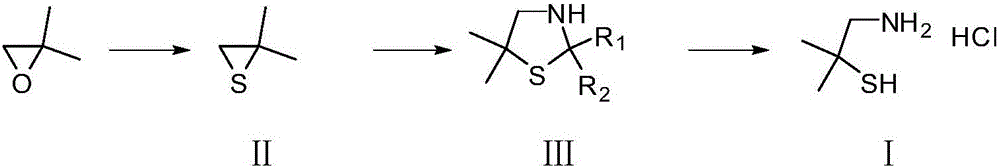
Method for synthesizing 2,2-dimethyl cysteamine hydrochloride
A technology of dimethylcysteine hydrochloride and dimethyl, which is applied in the field of synthesizing 2,2-dimethylcysteine hydrochloride, can solve the problem of harsh chlorination and reduction reaction conditions and high reagent prices. It is suitable for industrial production and other problems, and achieves the effects of low requirements for reaction equipment, shortened reaction time, and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042]
[0043] Add ammonia water (68.0 g) and formaldehyde aqueous solution (113.6 g) into a three-necked flask, raise the temperature to 50-55° C., and add dimethylsulfide (44.0 g) dropwise. After the dropwise addition was completed, the reaction was incubated for 5 hours. The reaction solution was left to separate and separated, the water layer was discarded, the organic layer was collected, and the low boilers were removed under reduced pressure to obtain a colorless liquid, and then the crude product was distilled under reduced pressure to obtain 26.3 g of 5,5-dimethylthiazolidine with a yield of 45.0%. Content 99.0%.
[0044] 1HNMR (CDCl3, 400MHz) δ: 4.31(m, 2H), 2.38(s, 1H), 2.05(m, 2H), 1.45(s, 6H).
[0045] MS: [M+1]+=118.2
[0046]
[0047] Add the above-mentioned 5,5-dimethylthiazolidine and concentrated hydrochloric acid (120.5g) into a three-necked flask in turn, raise the temperature to 95°C for reaction, and at the same time distill off the low boilers u...
Embodiment 2
[0051]
[0052]Ammonia water (68.0 g) and acetone (43.5 g) were added into a three-necked flask, the temperature was raised to 50-55° C., and dimethylsulfide (44.0 g) was added dropwise. After the dropwise addition was completed, the reaction was incubated for 5 hours. The reaction solution was allowed to stand for stratification, the water layer was discarded, the organic layer was collected, and the low boilers were removed under reduced pressure to obtain a colorless liquid, and then the crude product was distilled under reduced pressure to obtain 69.2 g of 2,2,5,5-tetramethylthiazolidine. The rate is 95.2%, and the content is 97.0%.
[0053] 1HNMR (CDCl3, 400MHz) δ: 3.02(s, 2H), 2.63(s, 1H), 1.59(s, 6H), 1.44(s, 6H).
[0054] MS: [M+1]+=145.9
[0055]
[0056] Add the above-mentioned 2,2,5,5-tetramethylthiazolidine and concentrated hydrochloric acid (255.0g) into a three-necked flask in turn, raise the temperature to 95°C for reaction, and at the same time distill ...
Embodiment 3
[0060]
[0061] Add ammonia water (68.0 g) and butanone (54.0 g) into a three-necked flask, raise the temperature to 50-55° C., and add dimethylsulfide (44.0 g) dropwise. After the dropwise addition was completed, the reaction was incubated for 5 hours. The reaction solution was allowed to stand for stratification, the water layer was discarded, the organic layer was collected, and the low boilers were removed under reduced pressure to obtain a colorless liquid, and then the crude product was distilled under reduced pressure to obtain 2,5,5-trimethyl-2-ethylthiazolidine 73.4 g, yield 92.3%, content 97.3%.
[0062] 1HNMR (CDCl3, 400MHz) δ: 3.08-2.93(m, 2H), 2.56(s, 1H), 1.92-1.71(m, 2H), 1.53(s, 3H), 1.45(s, 3H), 1.39(s , 3H), 1.01(t, J=7.6Hz, 3H)
[0063] MS: [M+1]+=160.2
[0064]
[0065] Add the above-mentioned 2,5,5-trimethyl-2-ethylthiazolidine and concentrated hydrochloric acid (296.7g) into a three-necked flask in turn, raise the temperature to 95°C for reaction...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com